Yttrofluorite and Chlorophane from Norway
Contributed by: Michael Crawford
Date: Dec 16th, 2025
Locality: Innhavet, Hamarøy, Nordland, Norway (See on Mindat)
Size: 5.5 x 9.5 cm
Description:
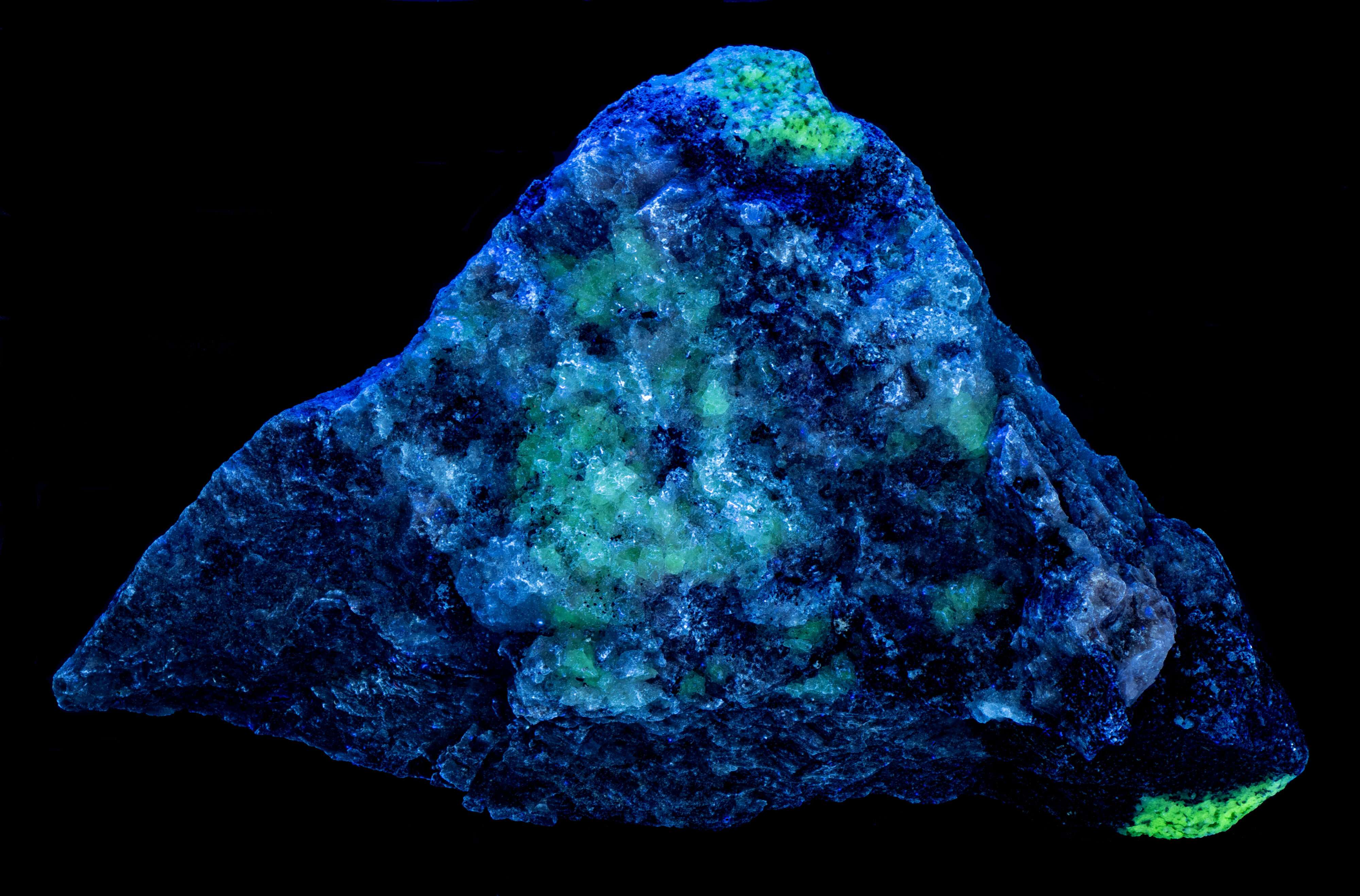
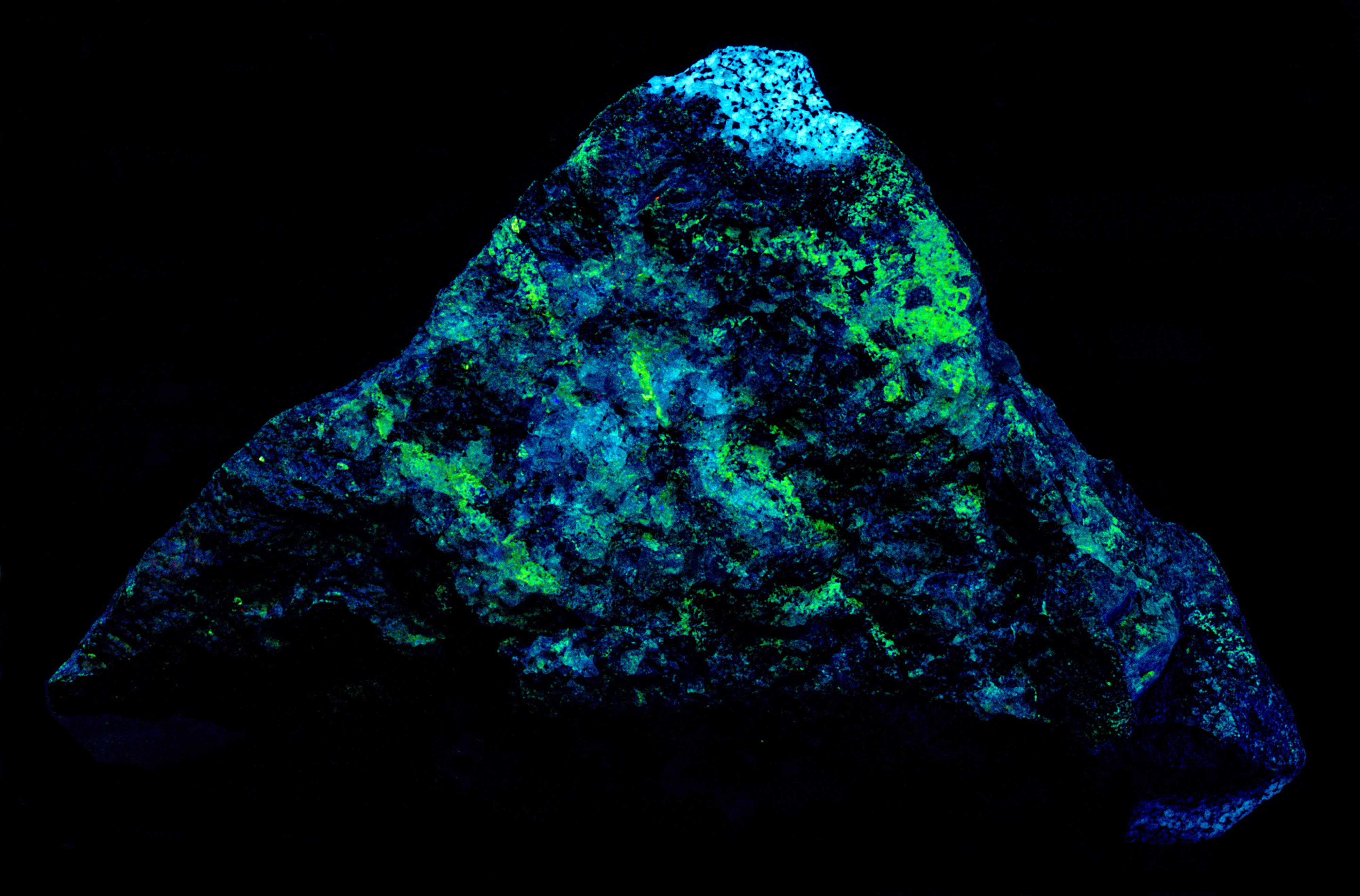
A specimen from Innhavet, Drag, Nordland, Norway. The specimen contains the fluorescent minerals yttrofluorite, fluorite var. chlorophane, albite and the non-fluorescent mineral allanite-(Ce). Yttrofluorite ((Ca1-xYx)F2-x) is a variety of fluorite containing an appreciable amount of trivalent yttrium (Y3+) taking the place of divalent calcium (Ca2+) cations in the fluorite structure. The allanite-(Ce) forms black reaction rim between the yttrofluorite and albite.
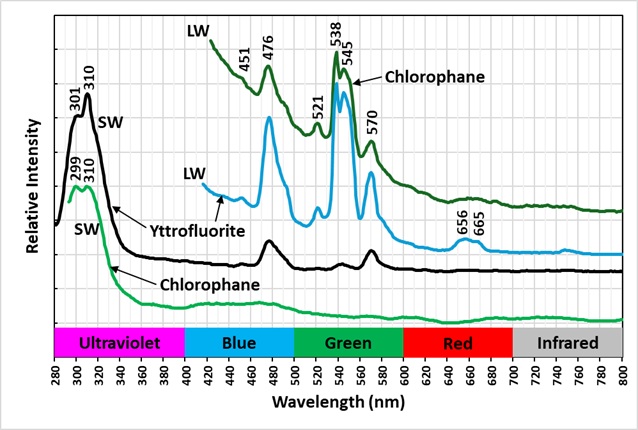
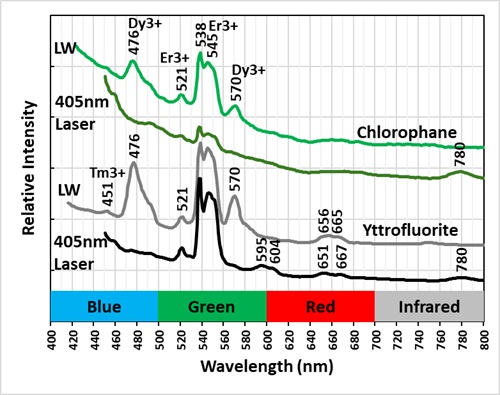
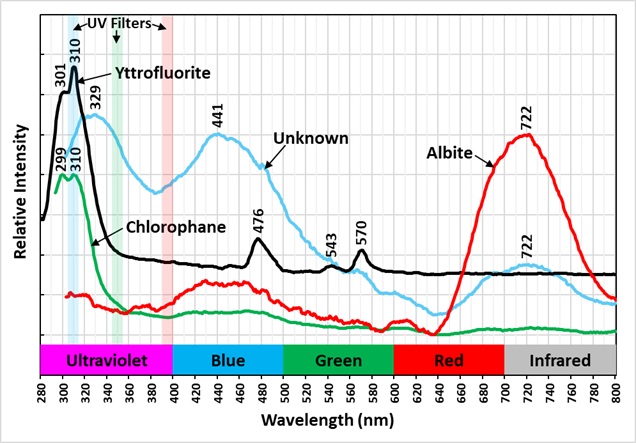
The emission spectra of yttrofluorite have numerous peaks indicative of rare earth element (REE) activation of the fluorescence. The shortwave emission has a very bright peak in the ultraviolet with a maximum at 310 nm and a secondary peak at 301 nm. This ultraviolet fluorescence is considerably brighter than the visible fluorescence of the yttrofluorite. The fluorescence is activated by yttrium (Y3+) replacing calcium. The other peaks in the visible are activated by other REE’s. Dysprosium (Dy3+), terbium (Tb3+), and erbium (Er3+) are likely REE activators.
The color of the yttrofluorite fluorescence changes with different wavelengths of illumination. It is green under longwave UV illumination and white under midwave and shortwave illumination. The green hue is created by a combination of a prominent peak composed of two sharp peaks with maxima at 538 nm and 545 nm and smaller peaks at 476 nm and 570 nm. The doublet peak is activated by terbium (Tb3+) and/or erbium (Er3+) and the peaks at 476 nm and 570 nm are activated by dysprosium. The peaks associated with dysprosium at 476 nm and 570 nm disappear in the spectrum activated by a 405 nm laser. The white color under shortwave illumination comes from dysprosium activated peaks at 476 nm and 570 nm. The green peaks at 538 nm and 545 nm activated by terbium/erbium are significantly reduced under shortwave light.
The chlorophane in this specimen is like the yttrofluorite in several aspects except that it has some green afterglow from exposure to shortwave light. The yttrofluorite has no afterglow. The fluorescence of the chlorophane is dimmer than the yttrofluorite fluorescence. The shortwave emission spectrum of chlorophane has a prominent peak in the ultraviolet with a doublet maximum at 299 nm and 310 nm like the yttrofluorite spectrum. However, chlorophane has no sharp peaks in the visible like the yttrofluorite spectrum. The longwave and 405 nm laser emission spectra of the chlorophane have the same sharp peaks activated by REE’s as the yttrofluorite spectra.
The emission spectra of the chlorophane and yttrofluorite indicate that the chemical activators for the fluorescence are similar. The afterglow and thermoluminescence of chlorophane is caused by lattice defects that do not occur in the yttrofluorite. The defect are missing fluorine ions. Electrons move into the fluorine voids and return to their ground state when heated and releasing a photon. The lattice defects are created by natural radioactivity from fluids that deposited the chlorophane and from radioactive minerals such as feldspars that form with the chlorophane.
The specimen also contains albite that fluoresces red under shortwave illumination. The albite emission spectrum peaks in the near infrared at 722 nm.
There is another fluorescent mineral in the specimen that is discriminated in the false color image of ultraviolet fluorescence. It appears as a green color in the image. Its emission spectrum has a broad ultraviolet peak with a maximum at 329 nm. It also has peaks at 441 nm and 722 nm. The mineral may be a feldspar and its ultraviolet fluorescence may be activated by cerium. The yttrofluorite appears light blue in the false color image and the chlorophane is blue gray in the image.
Summary of luminescence responses:
Yttrofluorite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Green
- Fluorescence under Shortwave (255nm LED) UV light: White
- Fluorescence under Longwave (365nm LED) UV light: Green
- Fluorescence under Shortwave (255nm LED) UV light: Green
- Afterglow after exposure to Shortwave (255nm LED) UV light: Green