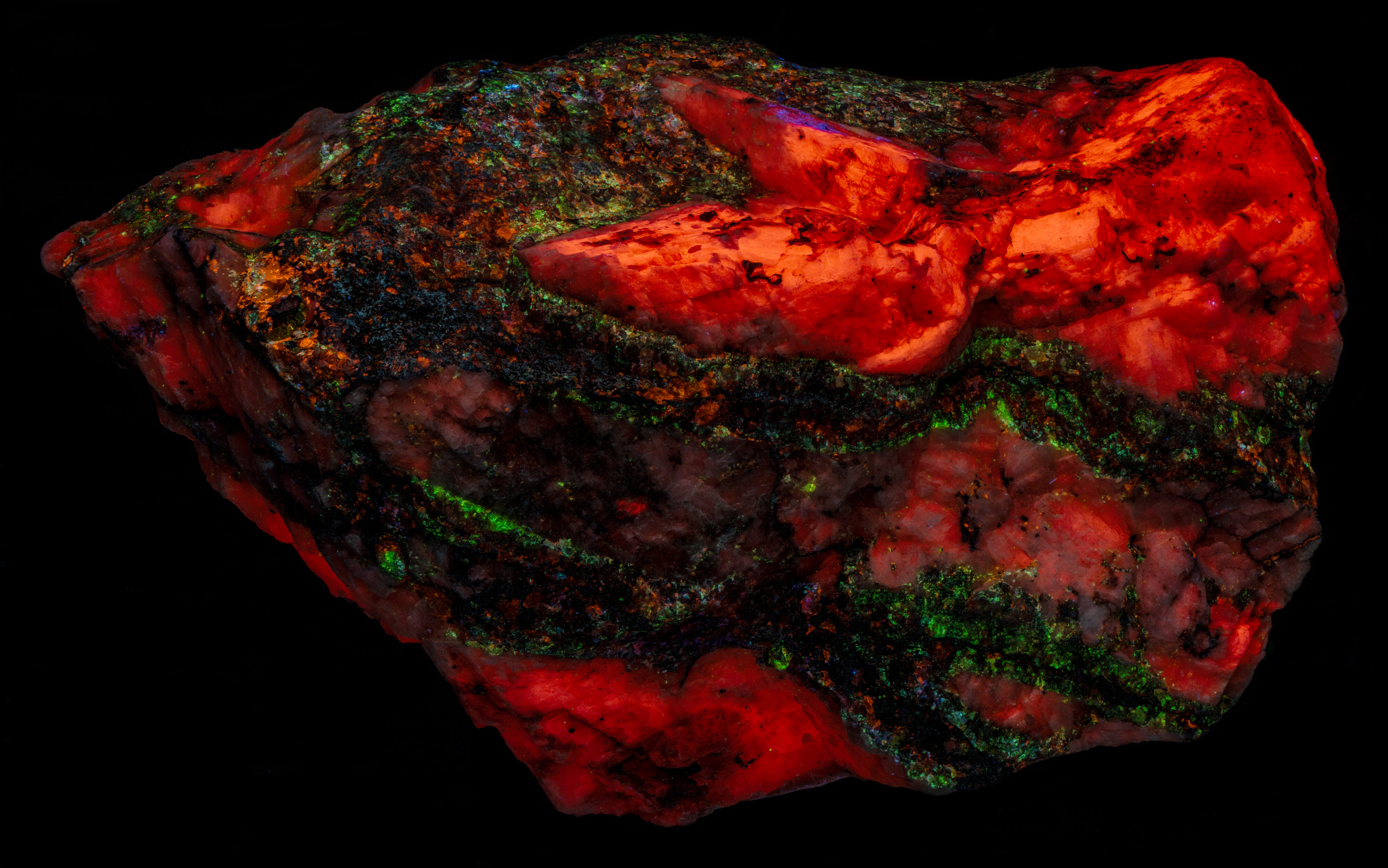
Green Fluorescent Sphalerite from Franklin, New Jersey
Contributed by: Michael Crawford
Date: Nov 29th, 2025
Locality: Franklin Mine, Franklin, Sussex County, New Jersey, USA (See on Mindat)
Size: 5 x 8 cm
Description:
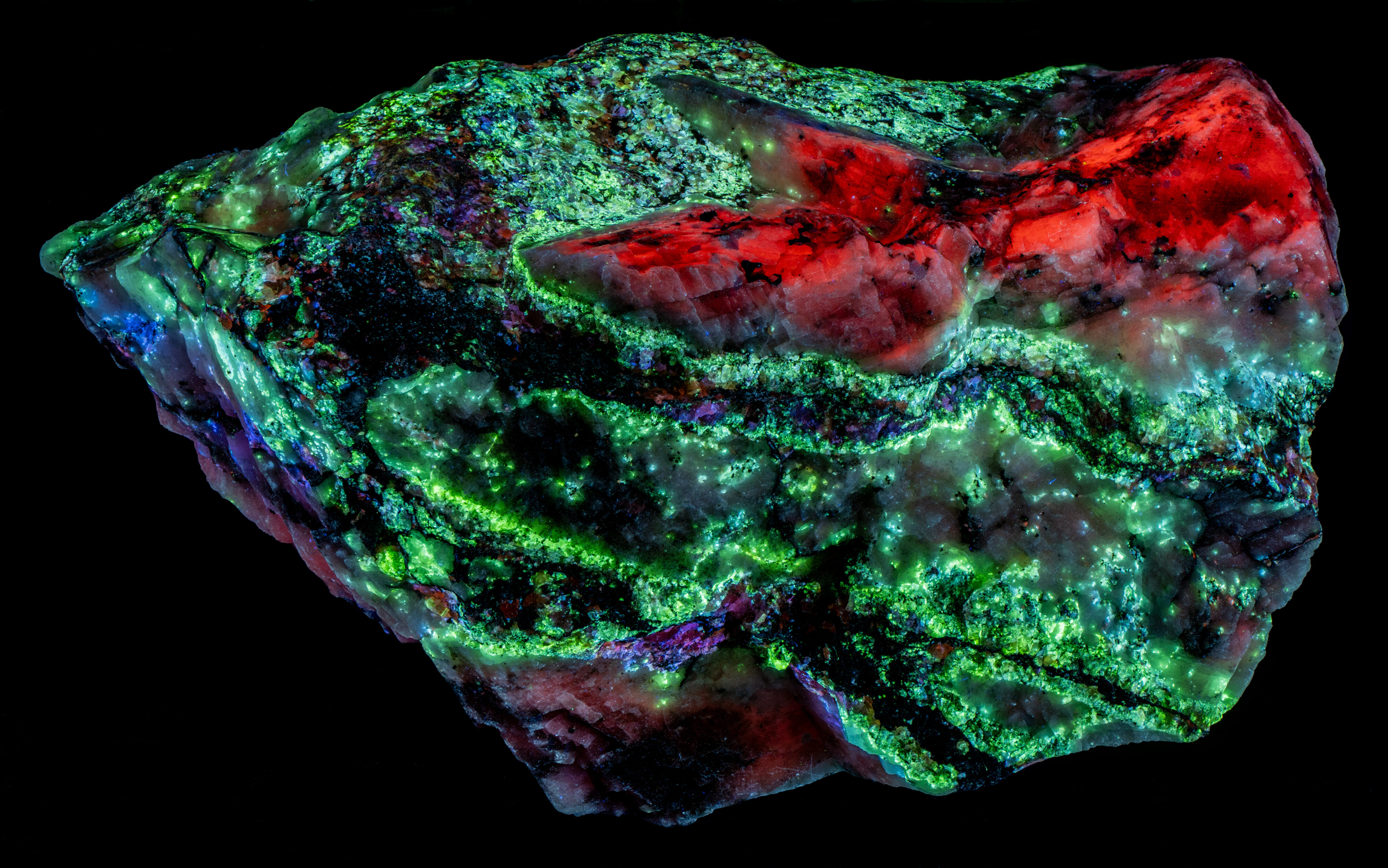
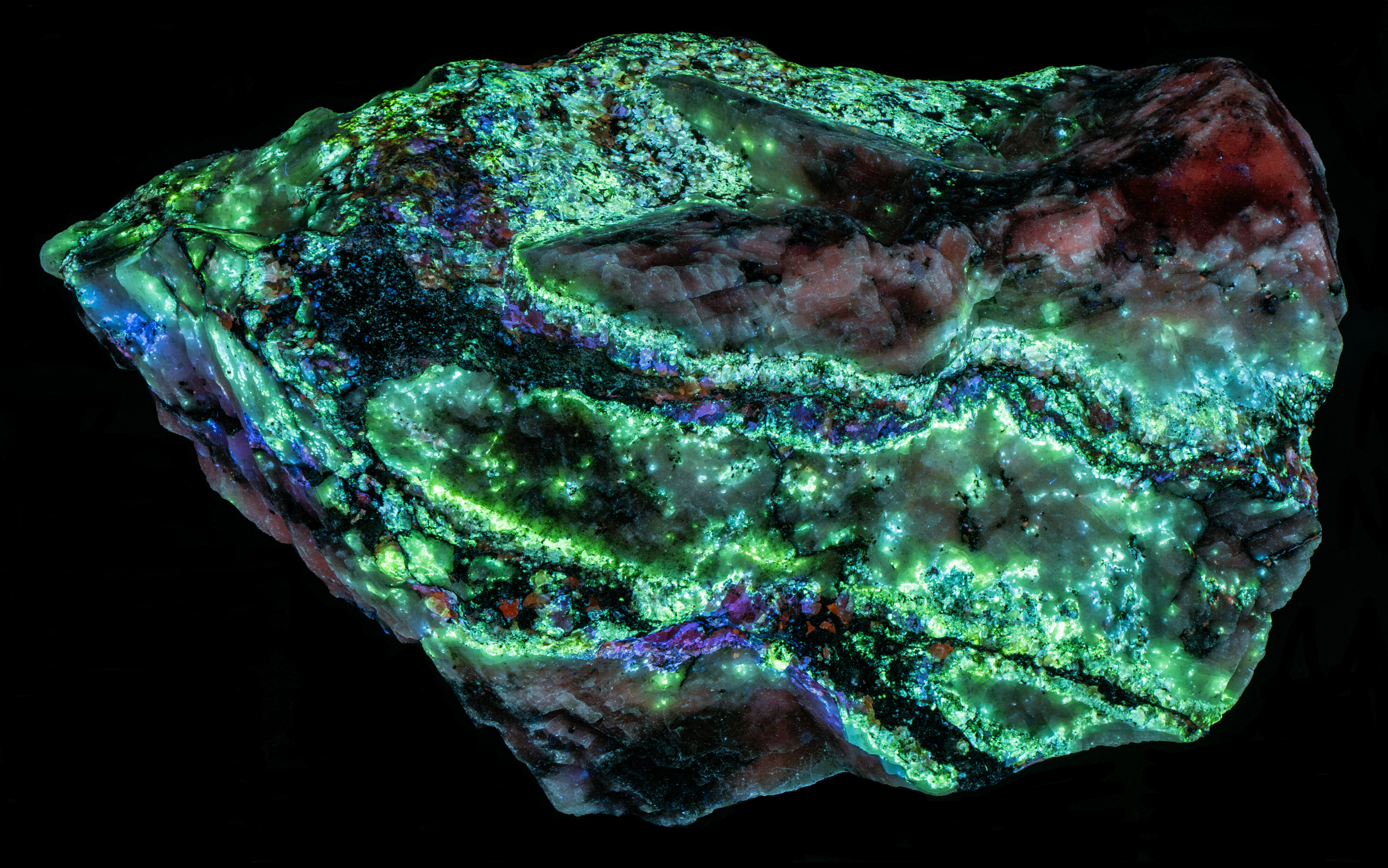
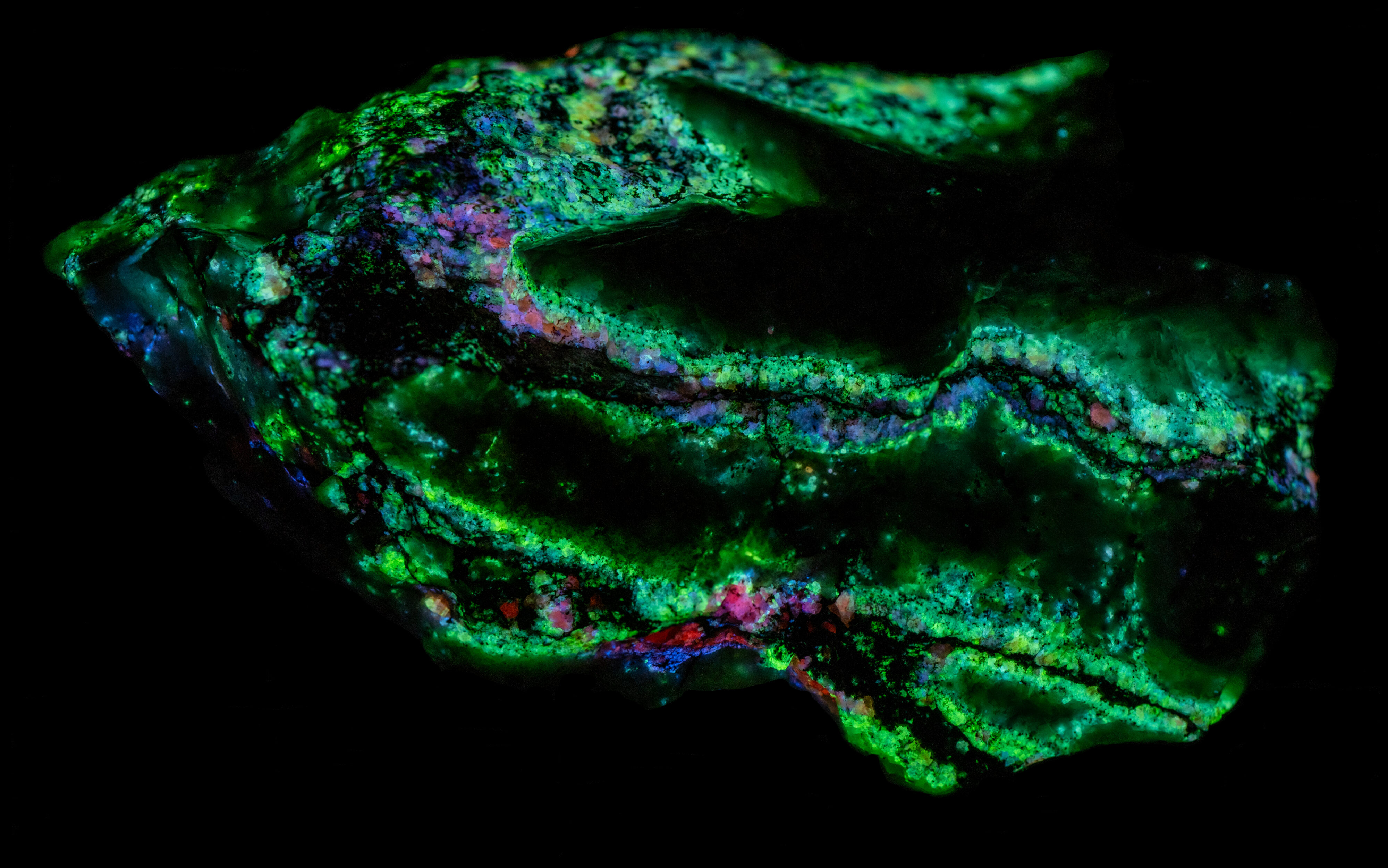
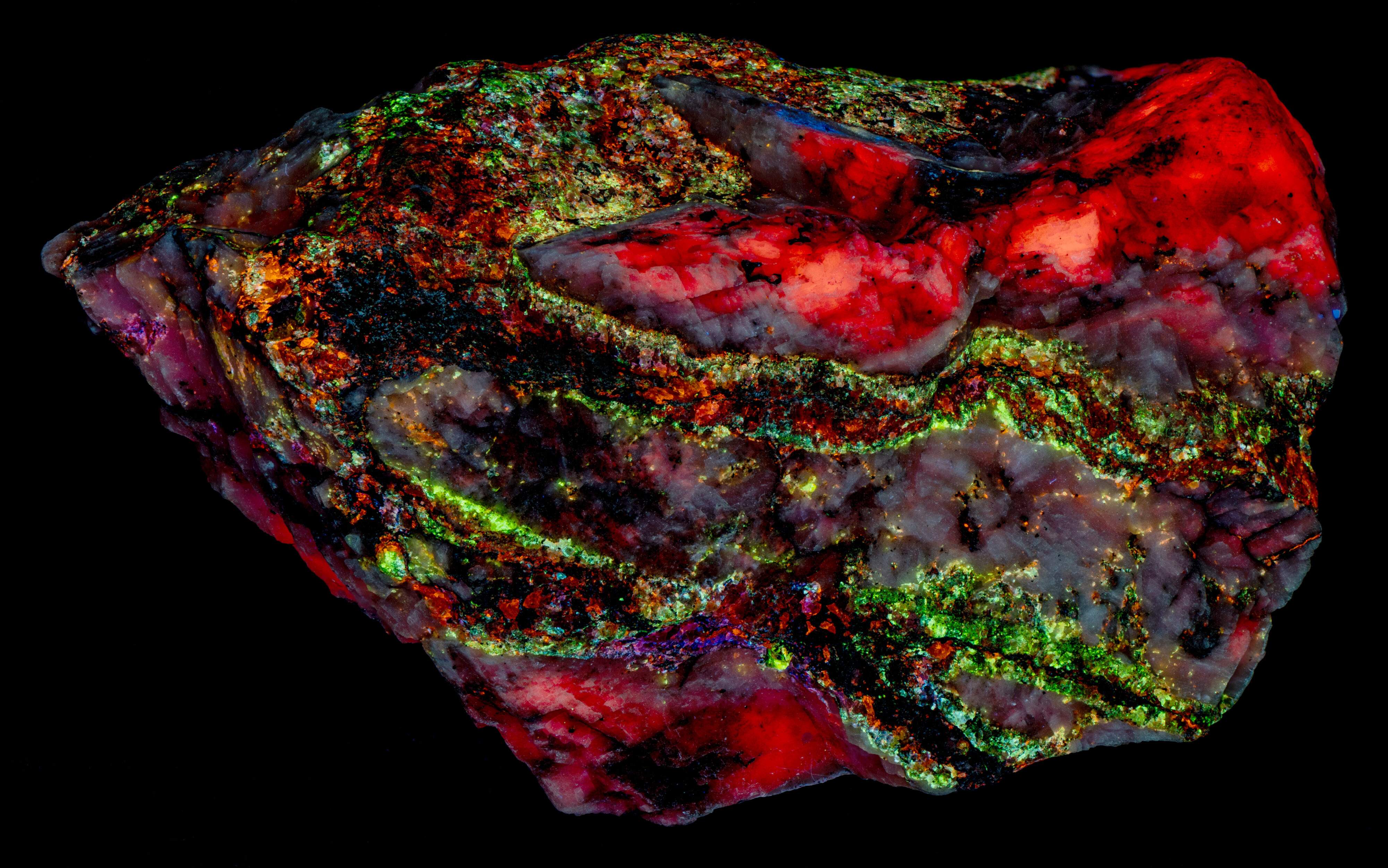
This is a Franklin, New Jersey specimen with veins of sphalerite cutting a calcite matrix. The sphalerite in the veins fluoresces several colors, blue green, blue, violet, and orange. The sphalerite fluoresces brightly under longwave UV illumination and has long lasting afterglow. The sphalerite fluorescence is considerably diminished under midwave and shortwave UV light and there is no afterglow. The blue green, fluorescent sphalerite occurs along the outer edges of the veins where the veins are in contact with the calcite matrix. The center of the veins is dominantly a black non-fluorescent mineral (perhaps non-fluorescent sphalerite) with blebs of pink, blue and orange fluorescent sphalerite.
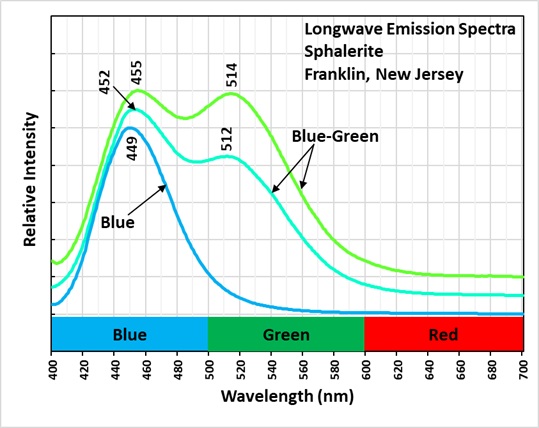
The longwave emission spectra of the blue green fluorescence has two peaks, one in the blue region with a maximum around 450-455 nm and a second in the green region with a maximum around 512 nm.
Green sphalerite fluorescence is caused by copper and aluminum substituting for two zinc ions. Copper is usually introduced into the sphalerite (ZnS) structure as Cu+. This is a different valence from Zn2+ so to get significant copper substitution there needs to be a way to balance charge. One way is to substitute Cu+ as well as Al3+ for two Zn atoms (Zn2+). The nominal Cu+ and Al3+ sum to a plus 4 the same as two Zn2+. Waychunas (2020) explained the details on how the copper-aluminum replacement activates the green sphalerite fluorescence.
The calcite matrix is brightest under shortwave light. Its red fluorescence is activated by manganese substituting for calcium.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Green
- Fluorescence under Longwave (365nm LED) UV light: Blue
- Fluorescence under Shortwave (255nm LED) UV light: Red