Color Changing Willemite from the Green Envy Mine, Arizona
Contributed by: Michael Crawford
Date: Oct 17th, 2025
Locality: Green Envy Mine, Vulture Mining District, Maricopa County, Arizona, USA (See on Mindat)
Size: 11 x 11.5 cm
Description:
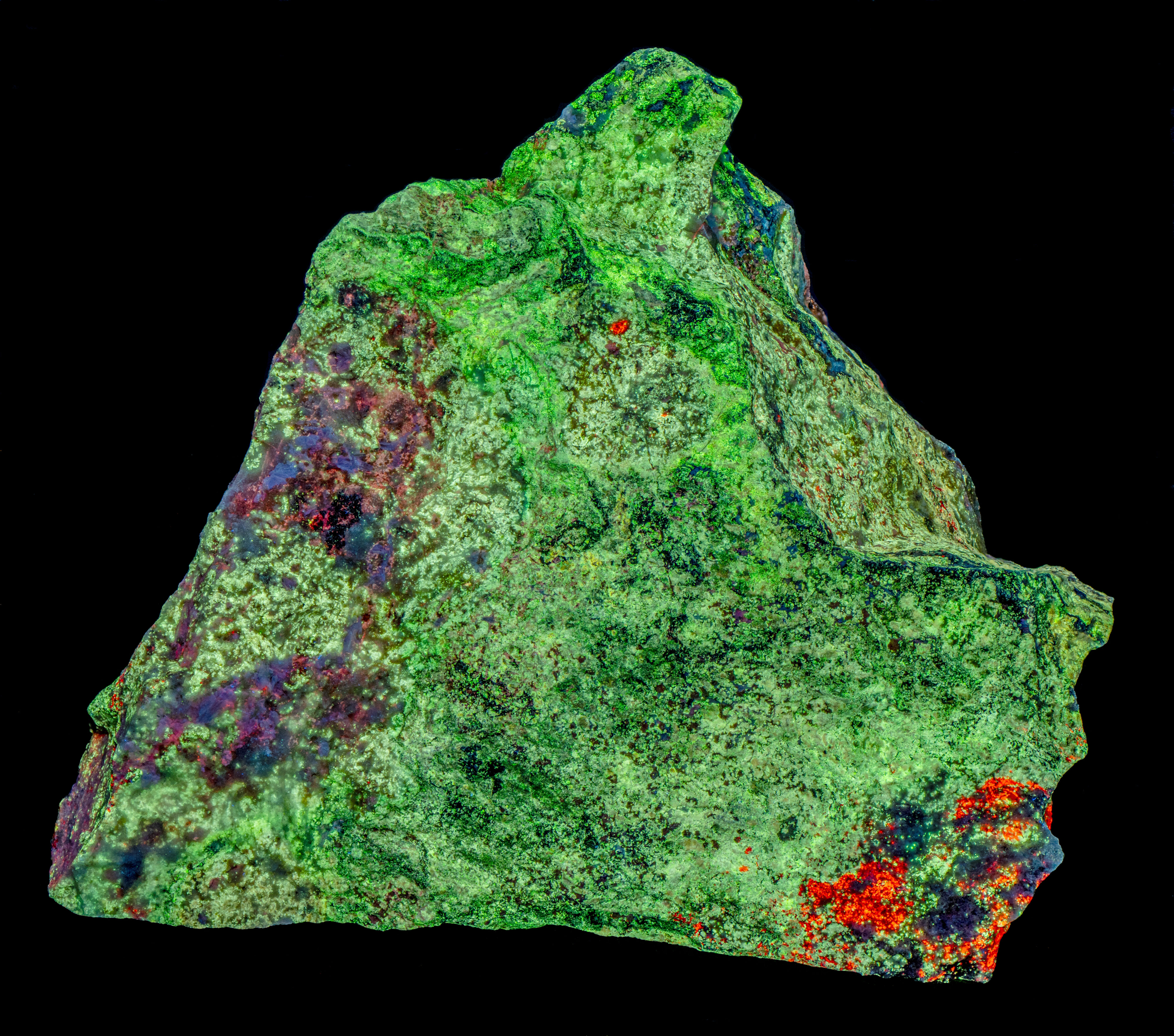
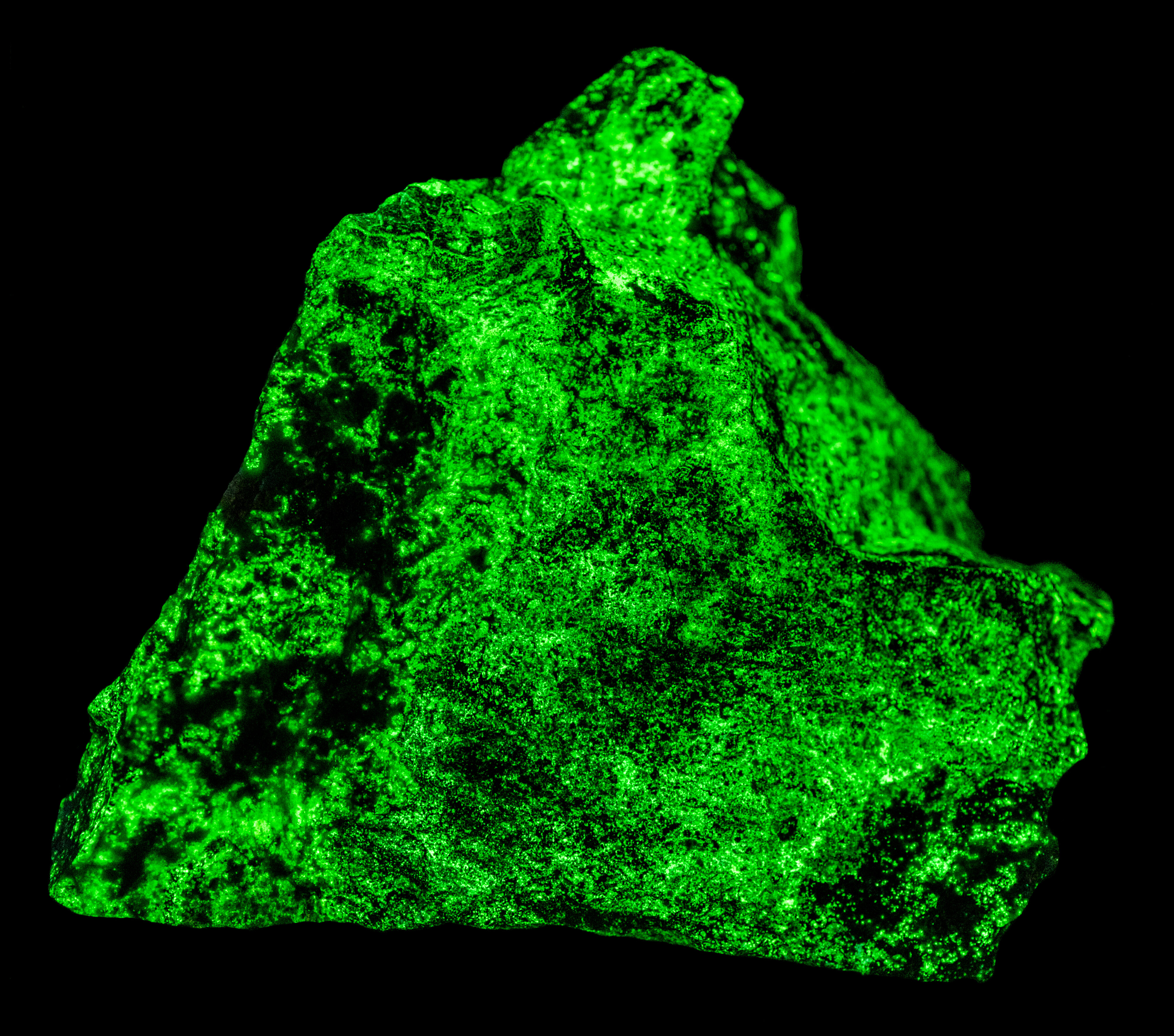
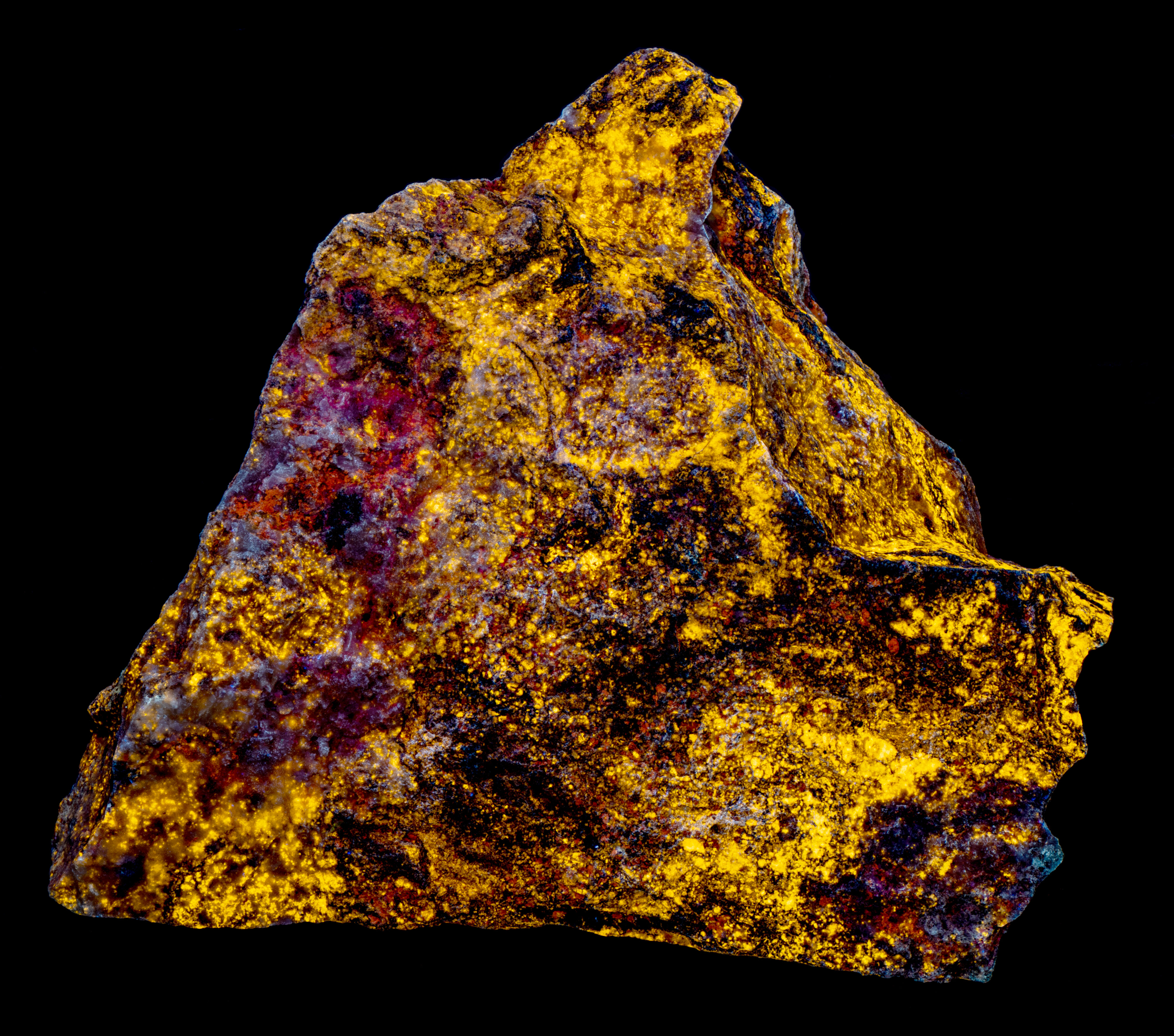
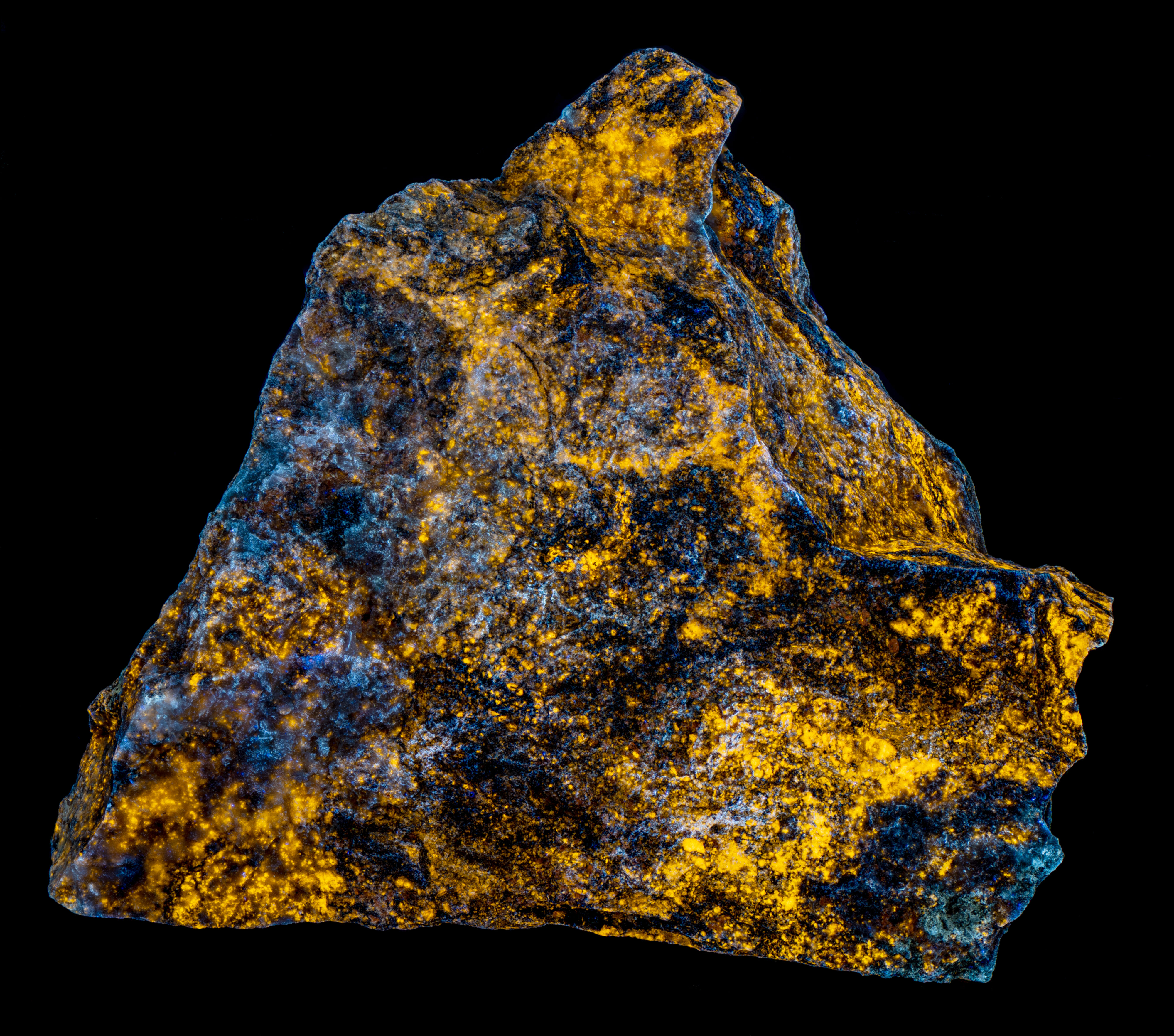
This is a willemite specimen that changes color under different UV wavelengths of illumination. It fluoresces yellow under longwave and midwave light and fluoresces various hues of green under shortwave light. The specimen also has long-lasting green afterglow from exposure to shortwave light and shorter afterglow from midwave light. The specimen comes from the Green Envy Mine, Maricopa County, Arizona. The specimen also contains some calcite and wickenburgite (CaPb3Al2Si10O24(OH)6) that are fluorescent. The wickenburgite fluoresces dull pink to red under midwave and shortwave light. The calcite glows bright red under shortwave light. There are also patches of unusual violet-blue, fluorescent calcite under midwave and shortwave light. Strong effervescence from a drop of acid confirms the calcite identification.
There are two mechanisms of luminescence occurring while the shortwave light strikes the willemite, fluorescence and thermoluminescence. The willemite fluoresces with electrons excited to a higher energy level and then emit green photons when the electrons return to the ground state. Some of the excited electrons are “trapped” by nearby arsenic atoms that substitute for silicon. Some trapped electrons return to the ground state while the UV light is on and some electrons can stay trapped for a long time after the UV light is turned off until there is enough thermal energy to push them out of the trap and back to the ground state of the Mn2+ atom.
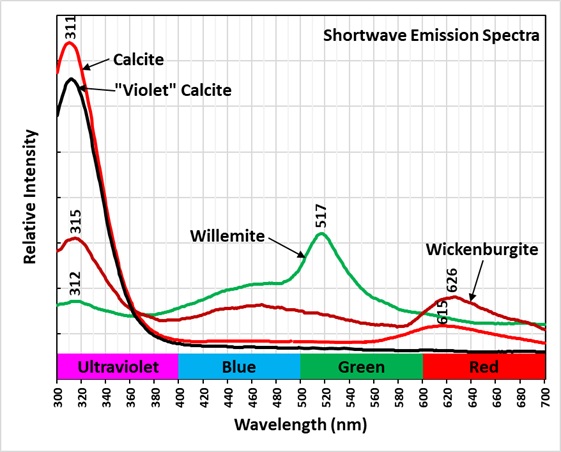
Shortwave emission spectra of the two types of calcite, willemite, and wickenburgite all contain a peak in the ultraviolet around 311 nm and 315 nm. The calcite peaks are much brighter than the wickenburgite and willemite peaks. The false color image of the ultraviolet fluorescence shows the areas of calcite as light blue patches. There is no distinction between the violet-blue and red calcite in this image like the difference seen in the true color image of visible shortwave fluorescence. The ultraviolet fluorescence of the willemite and wickenburgite is too dim to be detected in the ultraviolet image.
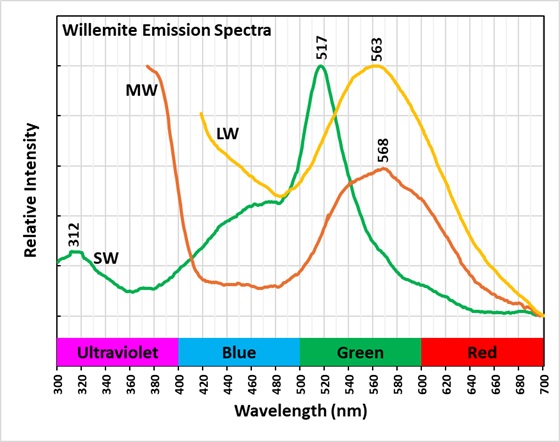
The shortwave emission spectrum of willemite is composed of two peaks in the visible. It has a broad peak that extends from 400 nm to 560 nm with a maximum around 470 nm. The second peak is much narrower and brighter than the broad peak. Its maximum is at 517 nm and is the green hue of the shortwave fluorescence. The peak is like the spectrum of Franklin, New Jersey willemite, but Franklin willemite typically has a maximum between 519 nm and 525 nm. This sharp peak is activated by manganese and the cause of the broad peak is unknown at this time. Another difference between this willemite and Franklin willemite is its yellow fluorescence under longwave and midwave UV light. The spectra of the yellow fluorescence peaks around 563 nm. The activation of this yellow fluorescence in unknown at this time.
Summary of luminescence responses:
Willemite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Yellow
- Fluorescence under Midwave (305nm LED) UV light: Yellow
- Fluorescence under Shortwave (255nm LED) UV light: Green