Mellite from Hungary
Contributed by: Michael Crawford
Date: Oct 8th, 2025
Locality: Csordakúti Mine, Bicske-Csordakút, Bicskei District, Fejér County, Hungary (See on Mindat)
Size: 7 x 9 cm
Description:
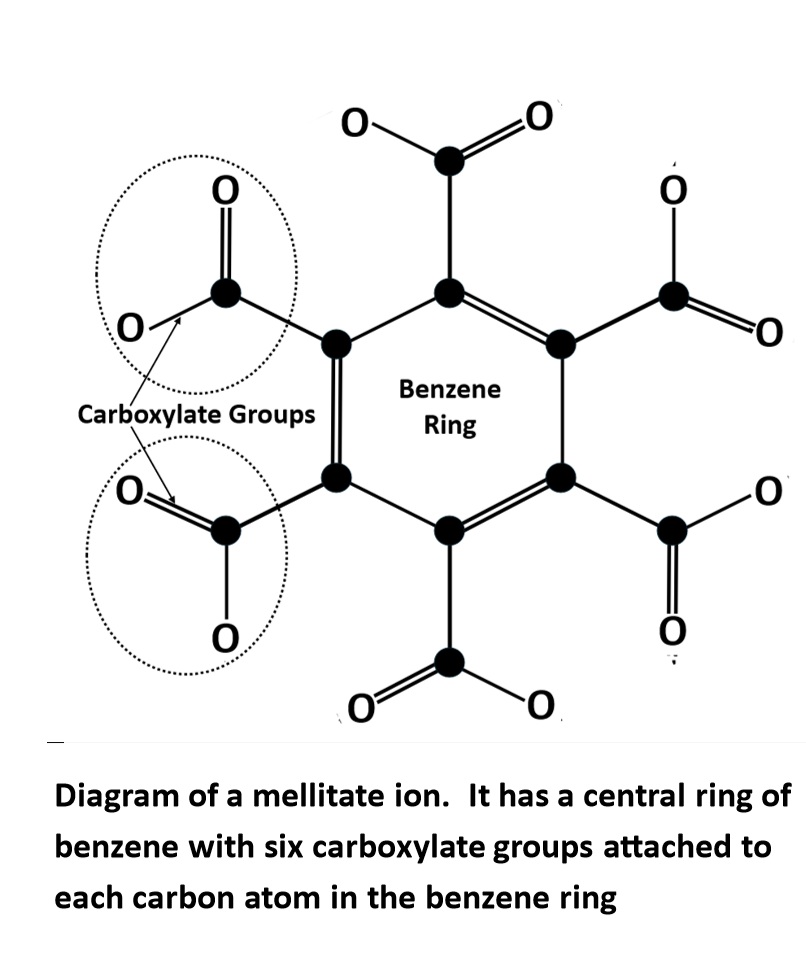
A mellite specimen from Csordakúti Mine, Hungary is a secondary organic mineral found in lignite deposits. Mellite is an aluminium mellitate hydrate (Al?C?(COO)?·16H?O). The mellitate ion, derived from mellitic acid, features a central benzene ring with six arms, each ending in a negatively charged carboxylate group giving it a 6- charge.
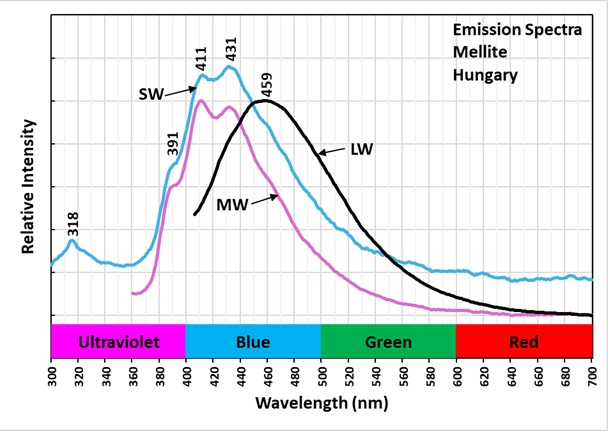
Mellite fluorescence has two different organic activators. One is intrinsic that produces blue fluorescence under midwave and shortwave UV illumination. This fluorescence is activated by the mellitate anion in mellite. The emission spectra of the midwave and shortwave have multiple peaks in the blue and ultraviolet regions. The two most prominent peaks occur at 411 nm and 431 nm. There are smaller peaks at 391 nm and 318 nm. The conjugated double bonds in the mellitate anion allow electrons to move to higher energy states. The electrons release photons when they return to the ground state. There is no afterglow from exposure to shortwave light. There is modest afterglow from midwave exposure. The midwave afterglow is likely from the second type of activator that causes the longwave fluorescence and afterglow.
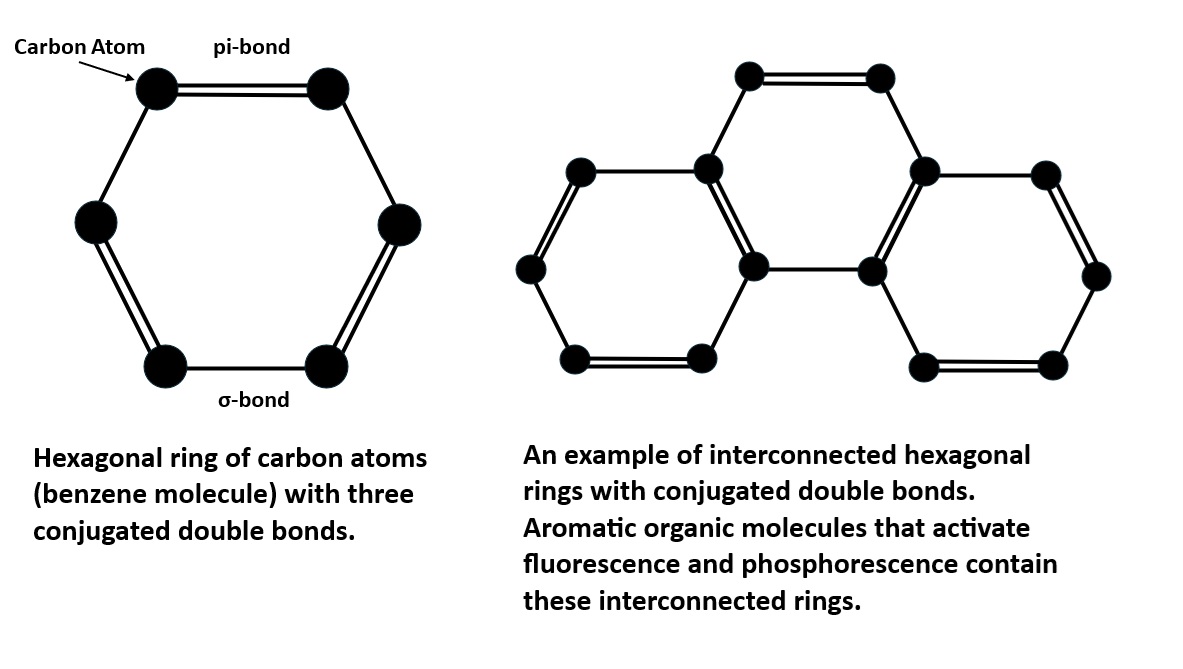
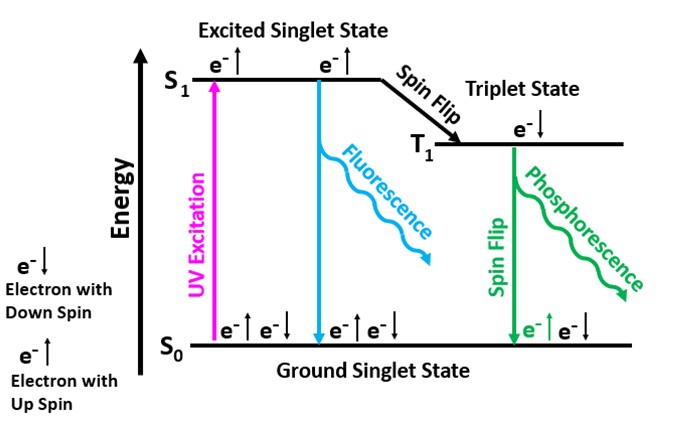
Aromatic organic molecules are the second type of activator responsible for the off-white longwave fluorescence and green afterglow. These aromatic compounds come from the conversion of plants to lignite. The aromatic molecules contain interconnected hexagonal benzene rings of carbon atoms with alternating single and double bonds. These conjugated double bands allow electrons to easily move from the ground singlet state to an excited singlet state at a higher energy level by exposure to UV light. According to quantum mechanics, an electron orbital can contain either one or two electrons in the ground singlet state. If there are two electrons in the orbital, they must have opposite spins. In aromatic molecules, the singlet state contains two electrons with opposite spins and when excited by UV light one electron moves to a higher energy level of an excited singlet state. Excited singlet states are short-lived, so most electrons return to the original ground singlet state, and the excess energy is emitted as a photon. The photon emission is the off-white fluorescence we see. The longwave emission spectrum of this fluorescence is a broad peak with a maximum at 459 nm.
Some electrons move from the excited singlet state to a slightly lower energy level known as the triplet state. The electron spin is flipped in the triplet state, and it now has the same spin as the electron left in the ground singlet state. Quantum mechanics forbids electrons of the same spin in the ground singlet state, so the electron in the triplet state must flip its spin to return to the ground singlet state. The electron must wait until the ambient thermal crystal lattice vibrations cause it to flip again before it can fall back down to its original state and emit a photon. The triplet state is at a lower energy level compared to the excited singlet state. Therefore, the energy of the photon emitted when the electron moves back to the ground singlet state from the triplet state is less. According to Planck’s Law, lower photon energy corresponds to a longer wavelength (green) for the phosphorescence. Temperature affects the time for the spin to flip and for the electron to return from the triplet state to its original singlet state. Cooling the crystal extends the phosphorescent time.
Summary of luminescence responses:
Mellite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: White
- Fluorescence under Midwave (305nm LED) UV light: Blue
- Fluorescence under Shortwave (255nm LED) UV light: Blue
- Afterglow after exposure to Longwave (365nm LED) UV light: Green
- Afterglow after exposure to Midwave (305nm LED) UV light: Green