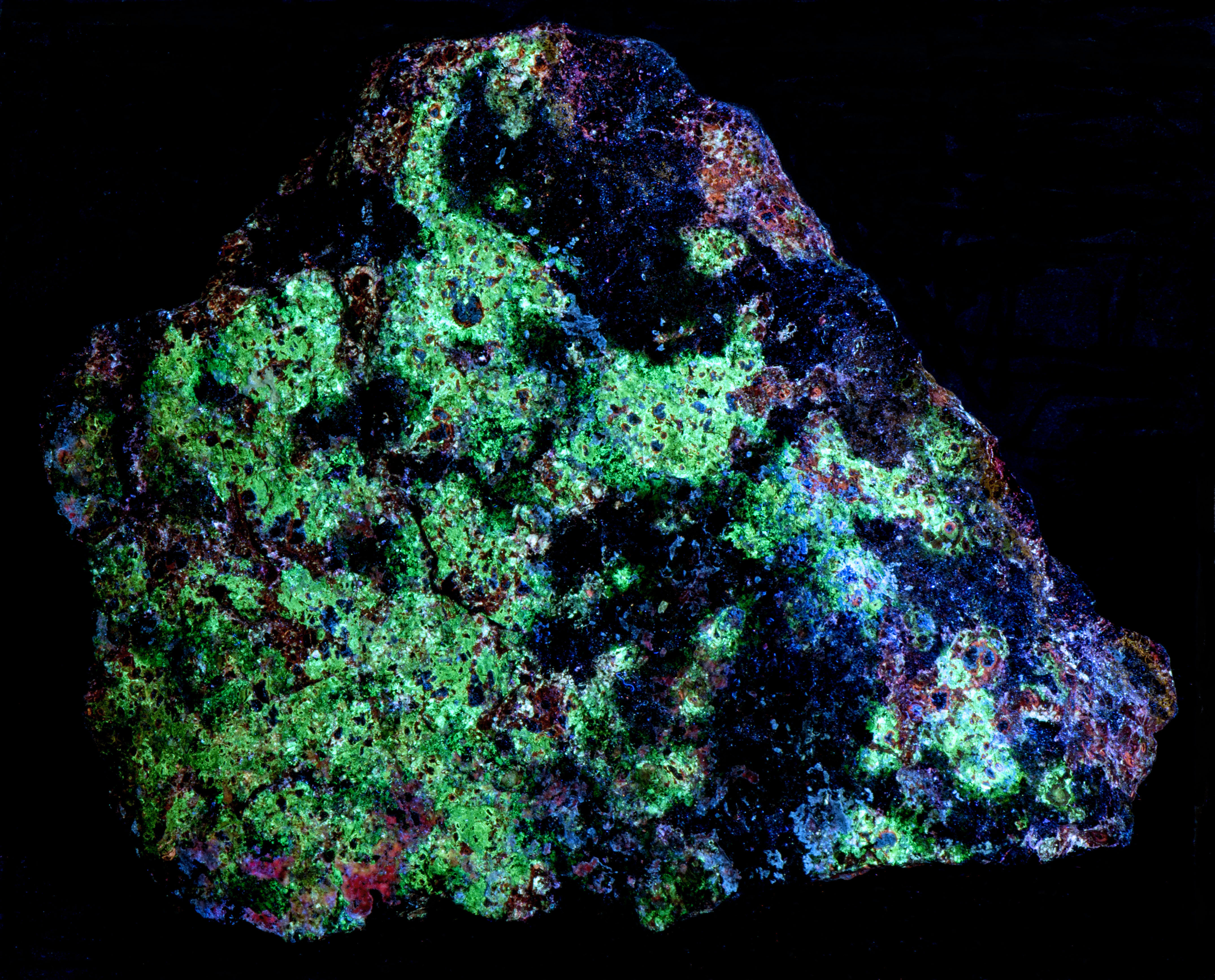
Green Fluorescent Sphalerite from Balmat, New York
Contributed by: Michael Crawford
Date: Oct 4th, 2025
Locality: Empire State No. 2 Mine, Balmat, Fowler, St. Lawrence County, New York, USA (See on Mindat)
Size: 8.5 x 10.5 cm
Description:
A sphalerite specimen from the Empire State No.2 Mine (ZCA #2 Mine), Balmat, St. Lawrence County, New York. Most of the sphalerite fluoresces bright green under all wavelengths of UV light. The green sphalerite has modest green afterglow after exposure to longwave light. There are also scattered grains of blue, fluorescent sphalerite under longwave light. There are small patches of orange, fluorescent sphalerite under longwave light and there are more patches of orange under midwave light. The fluorescence under shortwave light is similar to the midwave response.
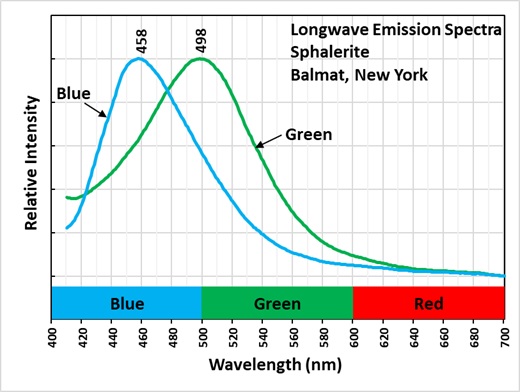
Sphalerite is a semi-conductor. The band gap between the valance band and the conduction band is narrow enough that electrons can move to the conduction band when sphalerite is exposed to UV light. Impurities such as copper, manganese, and silver can alter the band gap energy difference and activate a wide range of colors. The bigger energy gap between the valance band and conduction band with no impurities activates violet to blue fluorescence. Green sphalerite fluorescence is caused by copper and aluminum substituting for two zinc ions. Zinc ions have a 2+ charge, cuprous ions have a 1+ charge, aluminum ions have a 3+ charge. Two zinc ions (2 * 2+ = 4+) are replaced by a copper ion and an aluminum ion ((1+) + (3+) = 4+) in order to maintain charge balance. The longwave emission spectrum shows the green fluorescence peak at 498 nm in the Balmat specimen.
The blue fluorescence of the sphalerite has an emission spectrum with a peak at 458 nm. This blue fluorescence may also be caused by copper (Cu+) and aluminum impurities replacing zinc, but the replacement copper aggregates with interstitial copper in defect sites to activate blue fluorescence. Silver is also known to activate blue fluorescence, but the reported fluorescent peak is at 450 nm and the presence of silver is not reported at Balmat.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Green
- Fluorescence under Longwave (365nm LED) UV light: Blue