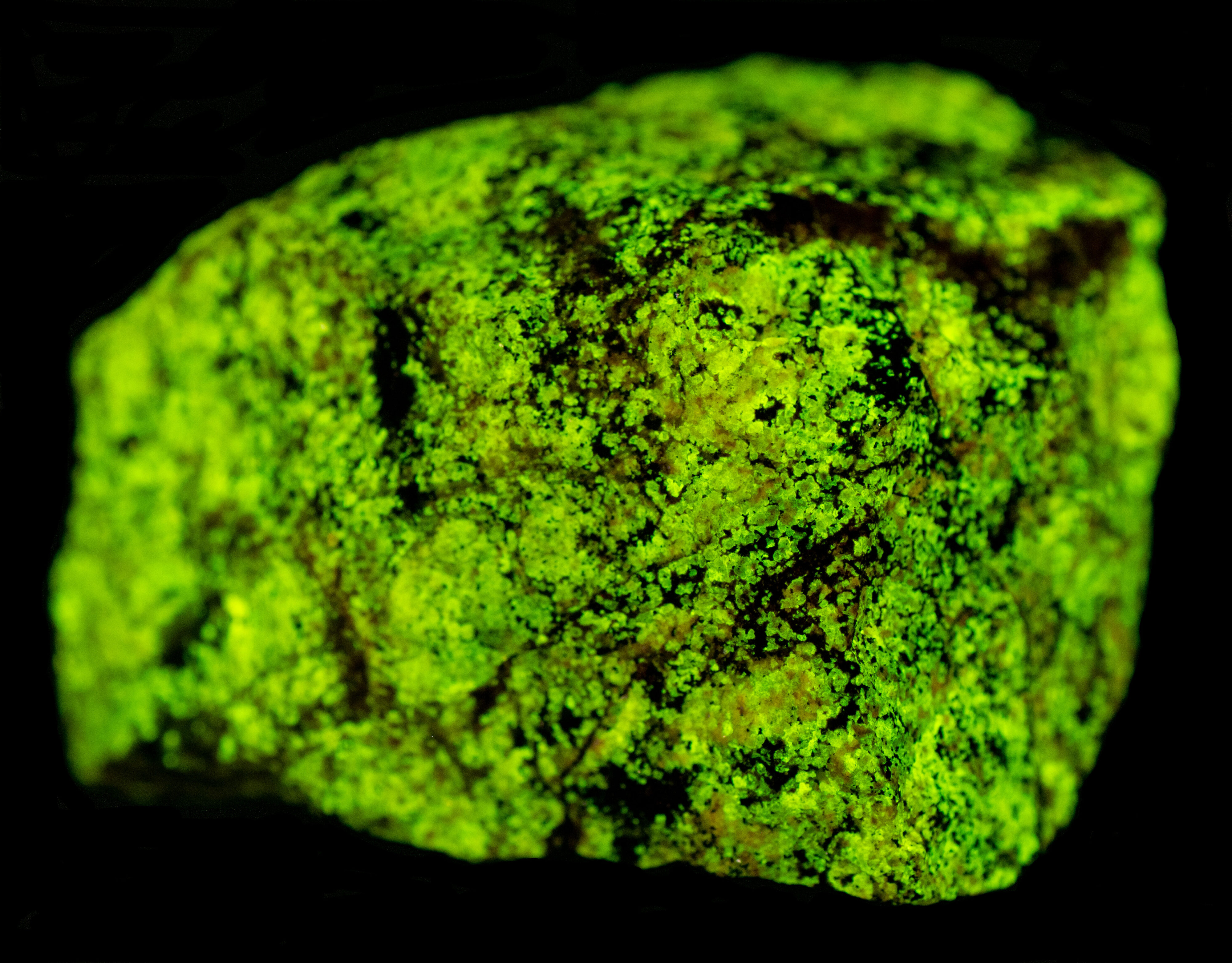
Green Fluoreescent Sphalerite from the Horn Silver Mine, Utah
Contributed by: Michael Crawford
Date: Oct 3rd, 2025
Locality: Horn Silver Mine, Frisco, San Francisco Mining District, Beaver County, Utah, USA (See on Mindat)
Size: 4 x 6 cm
Description:
A sphalerite (ZnS) specimen from the Horn Silver Mine, Beaver County, Utah. Green is an uncommon fluorescent color for sphalerite a zinc sulfide. Yellow to orange fluorescence is the most common fluorescent color of sphalerite. Sphalerite can also fluoresce blue, pink, red and near infrared. Different activators are responsible for the rainbow of colors of sphalerite fluorescence. Green sphalerite fluorescence is caused by copper and aluminum substituting for two zinc ions. The zinc ions have a 2+ charge, copper ions have a 1+ charge, aluminum ions have a 3+ charge. Two zinc ions (2 * 2+ = 4+) are replaced by a copper ion and an aluminum ion ((1+) + (3+) = 4+) in order to maintain charge balance. Details on how the copper-aluminum replacement activates the green sphalerite fluorescence are described by Waychunas in the 2020 FMS Journal.
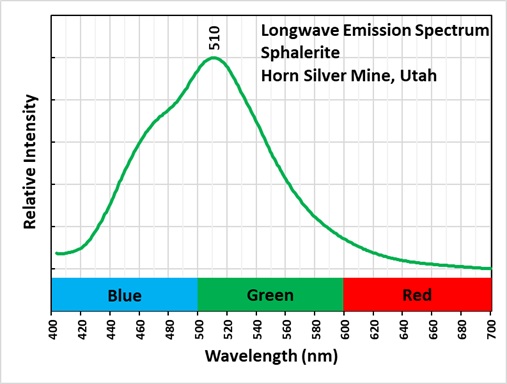
The longwave emission spectrum of the sphalerite has a peak at 510 nm and an inflection around 480 nm. This suggests that there are two types of activators present or a different co-activator. This specimen also has strong green afterglow from exposure to longwave light.
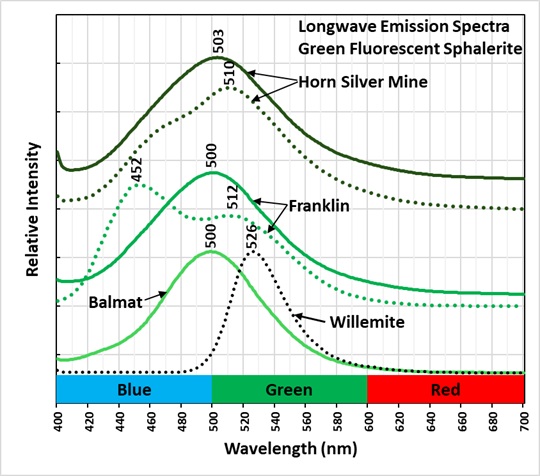
The green fluorescence of this specimen is slightly more yellow compared to other green, fluorescent sphalerites from Horn Silver Mine, Balmat, New York and Franklin, New Jersey. The longwave emission spectra of those sphalerites have peaks around 500 nm. The green fluorescence of willemite slightly more yellow compared to green sphalerite fluorescence. The peak of willemite fluorescence is at 526 nm.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Green
- Afterglow after exposure to Longwave (365nm LED) UV light: Green