Sphalerite var. Cleiophane from the Franklin Mine, Sussex County, New Jersey
Contributed by: Michael Crawford
Date: Sep 29th, 2025
Locality: Franklin Mine, Franklin, Sussex County, New Jersey, USA (See on Mindat)
Size: 7 x 10.5 cm
Description:
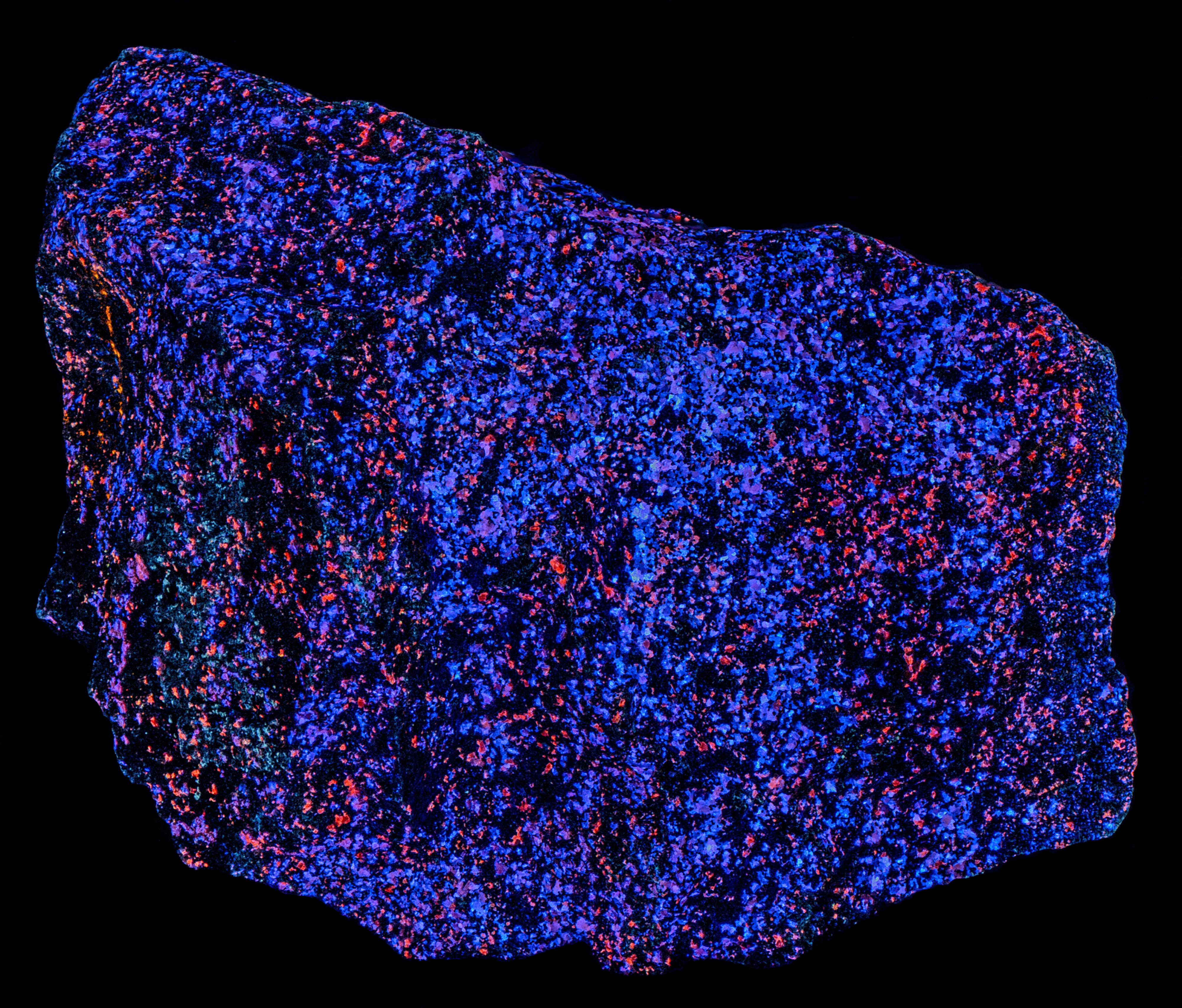
This is a specimen of sphalerite var. cleiophane from the Franklin Mine, Sussex County, New Jersey. The cleiophane occurs as scattered grains in a black matrix of franklinite or possibly magnetite. The specimen strongly attracts a magnet. Cleiophane is the sphalerite variety with low iron content.
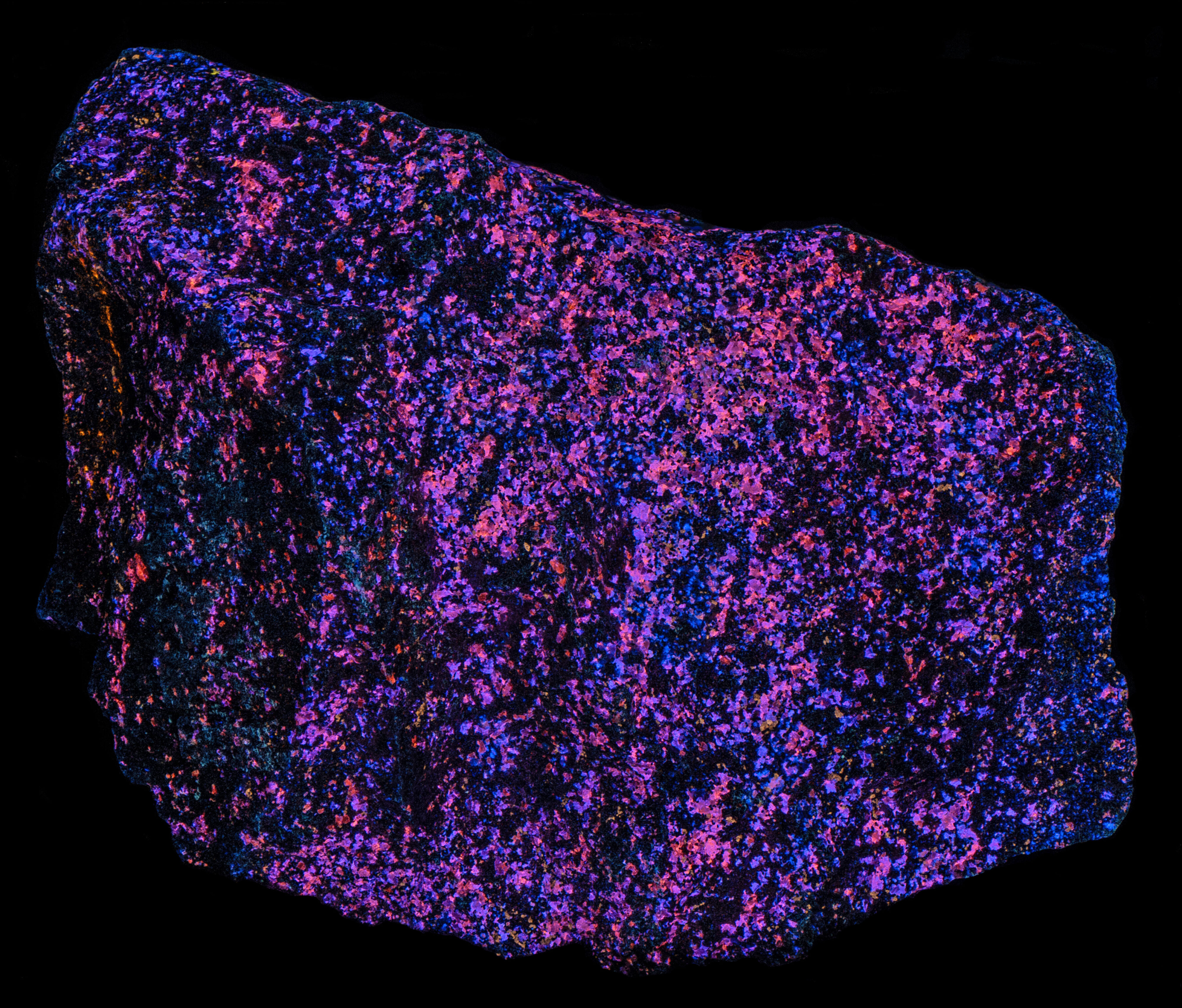
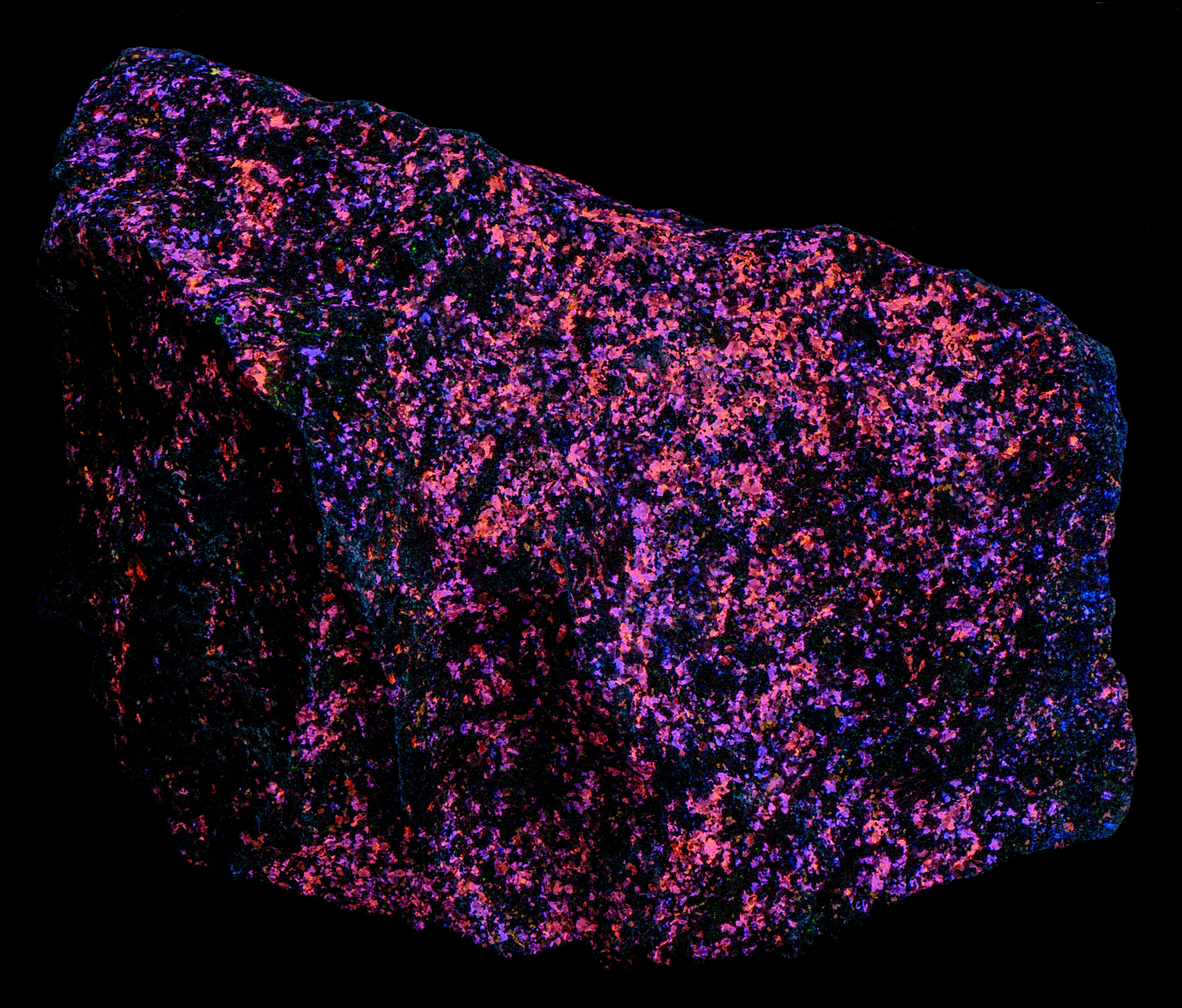
The most of the cleiophane fluoresces blue under longwave UV illumination. There are some grains that fluoresce orange. The amount of blue fluorescence decreases under midwave light and many of the cleiophane grains change to orange fluorescence. The fluorescence is mostly orange under shortwave light.
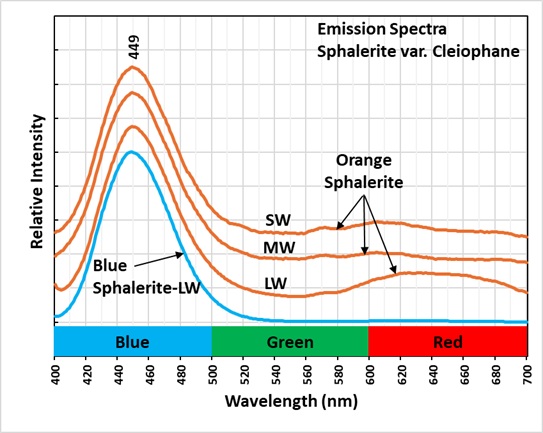
Sphalerite is a semi-conductor. The band gap between the valance band and the conduction band is narrow enough that electrons can move to the conduction band when sphalerite is exposed to UV light. When the electrons return to the valance band, blue photons are emitted. The emission spectrum of this blue fluorescence peaks at 449 nm. For more detail on semiconducting minerals and band transitions see the article by Glenn Waychunas, in the 2020 FMS Journal.
Manganese replacing zinc in the activates the orange fluorescence. It takes less energy to create this manganese activated fluorescence, so the light emitted is at a longer wavelength. Emission spectra of this orange fluorescence is a broad peak with maxima around 600 nm to 640 nm. The 449 nm peak is still present in the emission of the orange, fluorescent sphalerite.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Blue
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Fluorescence under Midwave (305nm LED) UV light: Orange
- Fluorescence under Shortwave (255nm LED) UV light: Orange