Agrellite and Albite from the Kipawa Alkaline Complex, Quebec, Canada
Contributed by: Michael Crawford
Date: Sep 21st, 2025
Locality: Kipawa alkaline complex, Les Lacs-du-Témiscamingue, Témiscamingue RCM, Abitibi-Témiscamingue, Québec, Canada (See on Mindat)
Size: 8.5 x 9 cm
Description:
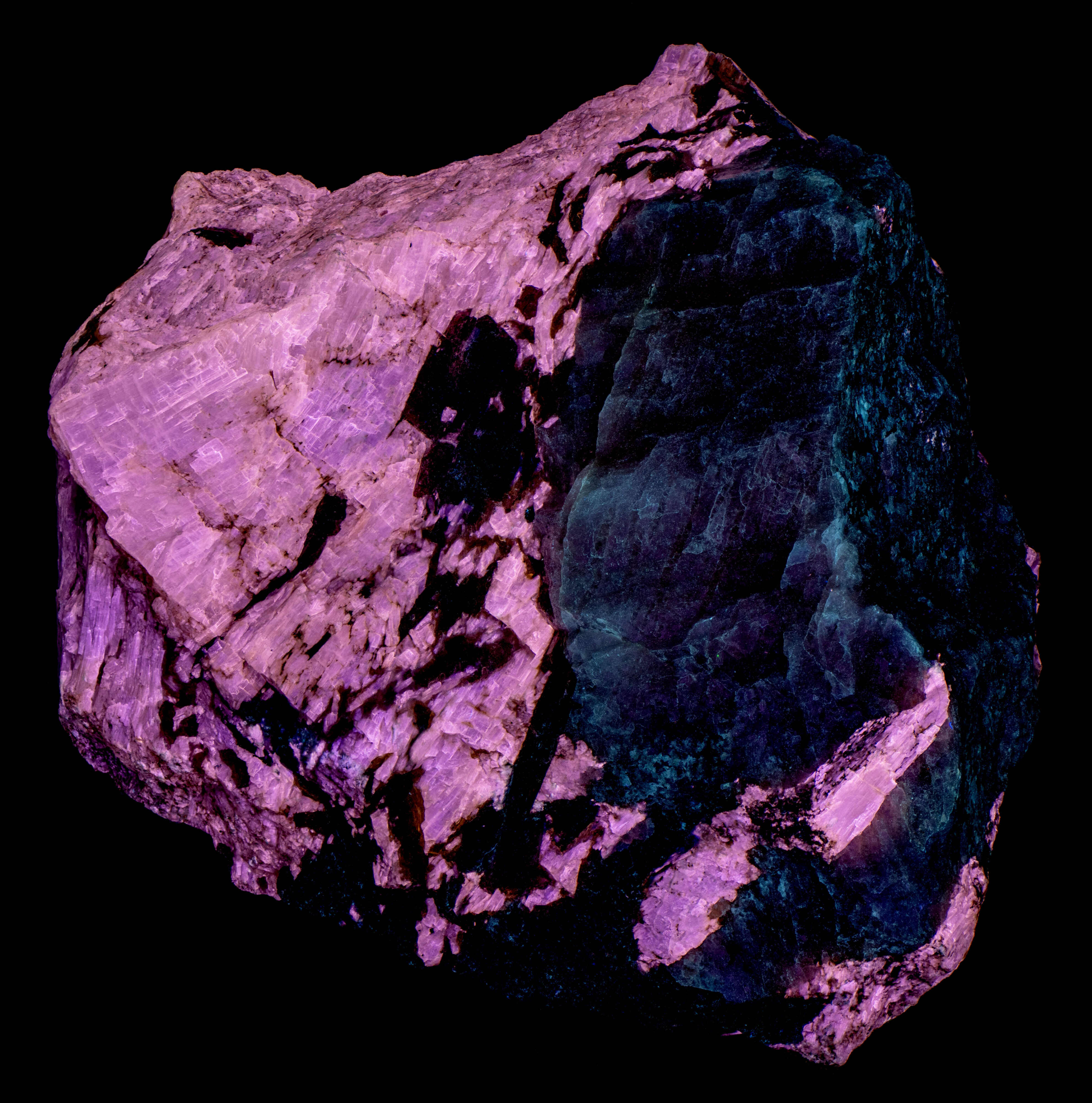
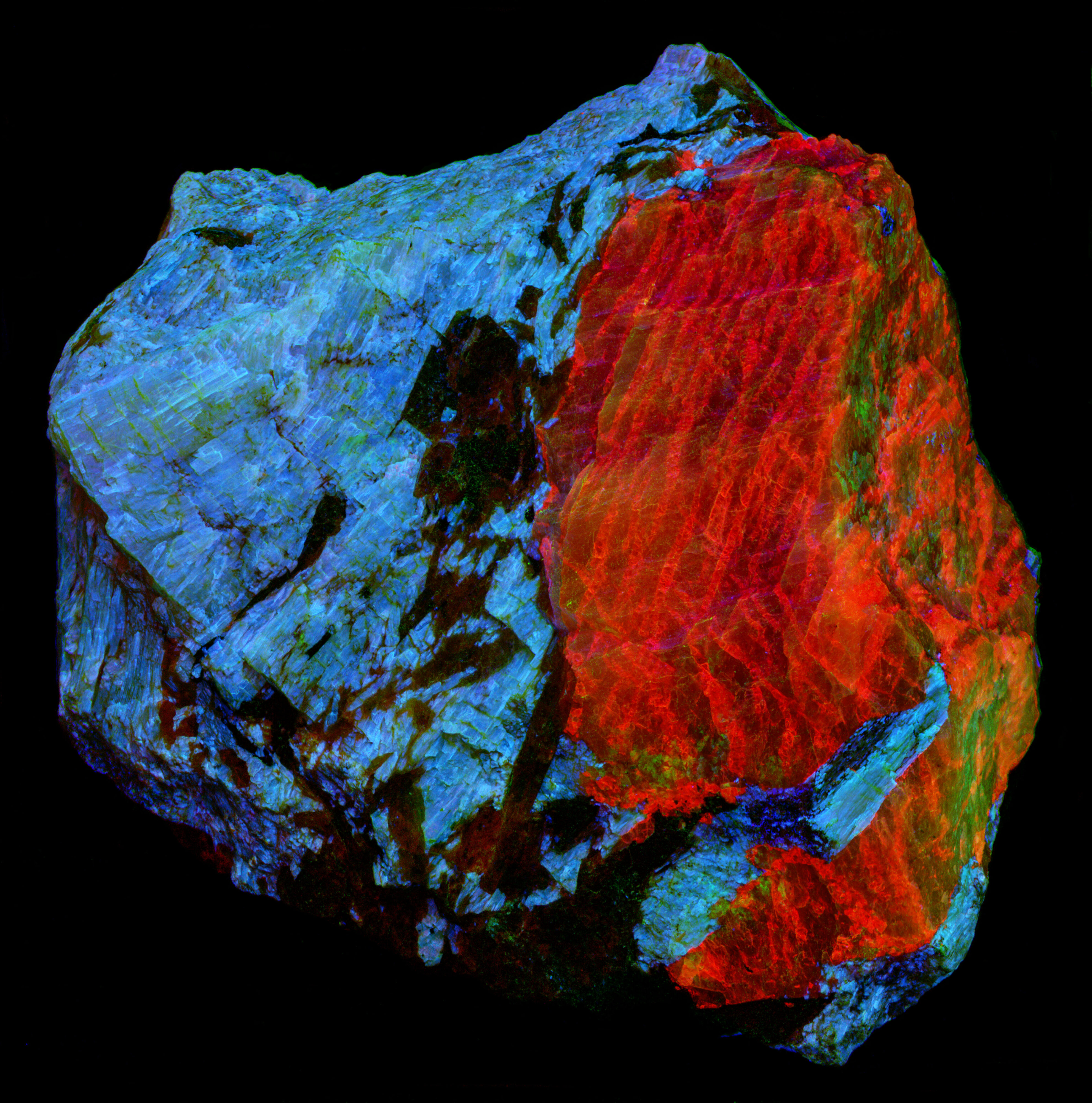
This is a specimen from the Kipawa Alkaline Complex, Les Lacs-du-Témiscamingue, Témiscamingue RCM, Abitibi-Témiscamingue, Québec, Canada. The specimen contains fluorescent agrellite (NaCa2Si4O10F), albite (Na(AlSi3O8)), and non-fluorescent red eudialyte (Na15Ca6Fe3Zr3Si(Si25O73)(O,OH,H2O)3(Cl,OH)2), and black amphibole.
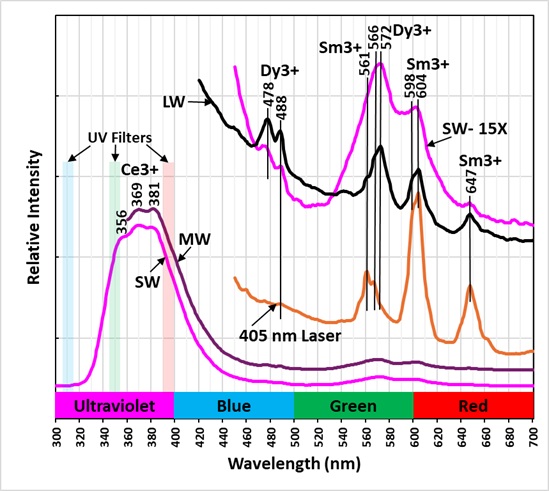
The shortwave emission peak of agrellite has several peaks that are activated by rare earth elements (REE). Agrellite has very bright fluorescence in the ultraviolet that is considerably brighter than its visible fluorescence. The ultraviolet fluorescence is activated by cerium (Ce3+) that replaces calcium in the agrellite structure. Cerium causes three sharp peaks at 356 nm, 369 nm and 381 nm. The vertically expanded plot of the shortwave emission spectrum in the visible shows several sharp peaks caused by rare REE replacing calcium:
Dysprosium (Dy3+) – 478 nm, 488 nm, and 572 nm
Samarium (Sm3+) – 561 nm, 604 nm, and 647 nm.
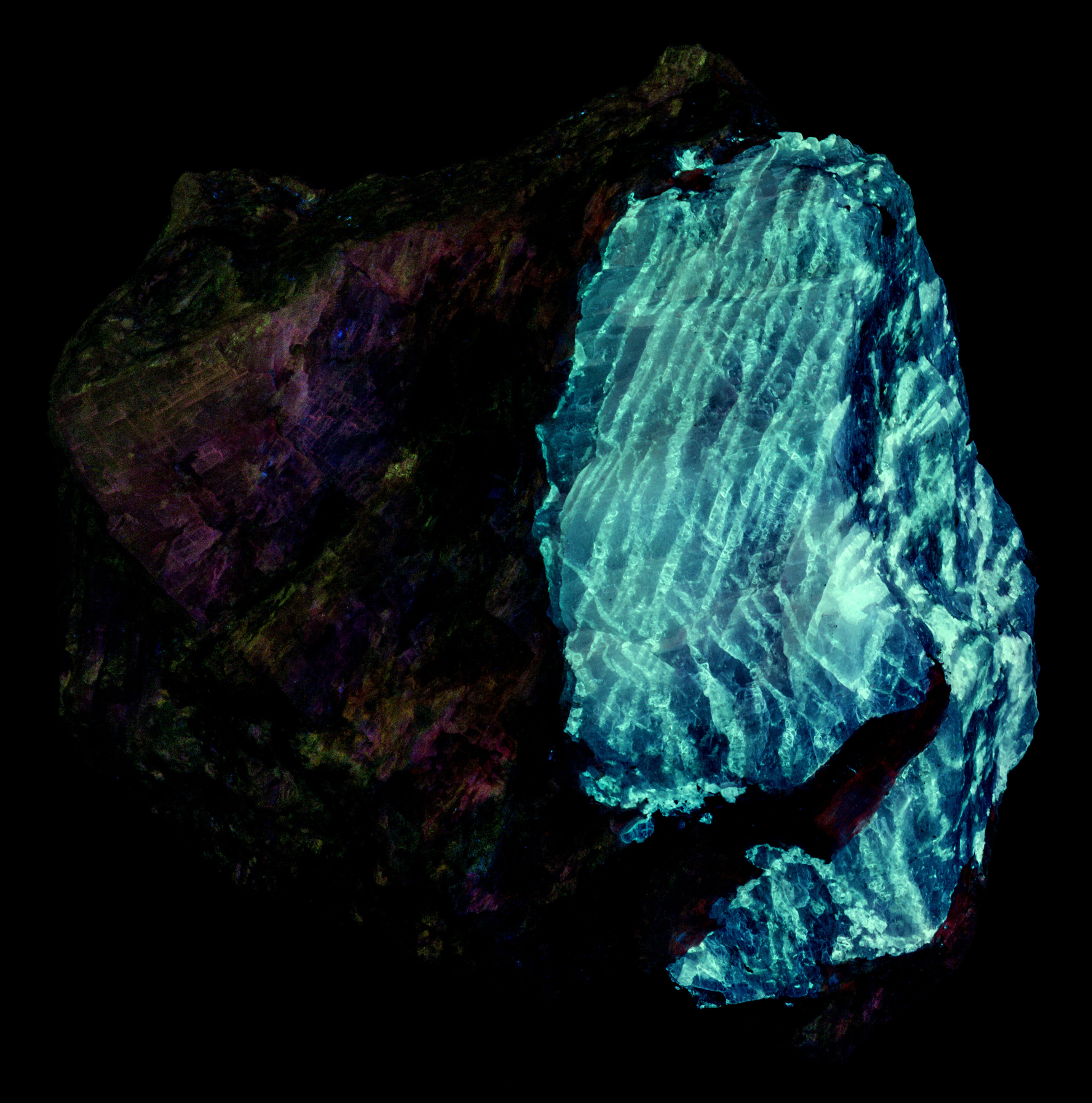
The pink fluorescent color of the agrellite under shortwave and midwave UV light is a result of the combination of shoulder of the ultraviolet fluorescence extending into the visible blue and the REE activated peaks. The agrellite fluorescence becomes orange under longwave UV light. There is no longer a blue contribution from the cerium activated ultraviolet fluorescence. The orange fluorescence comes from the dysprosium and samarium activated fluorescence.
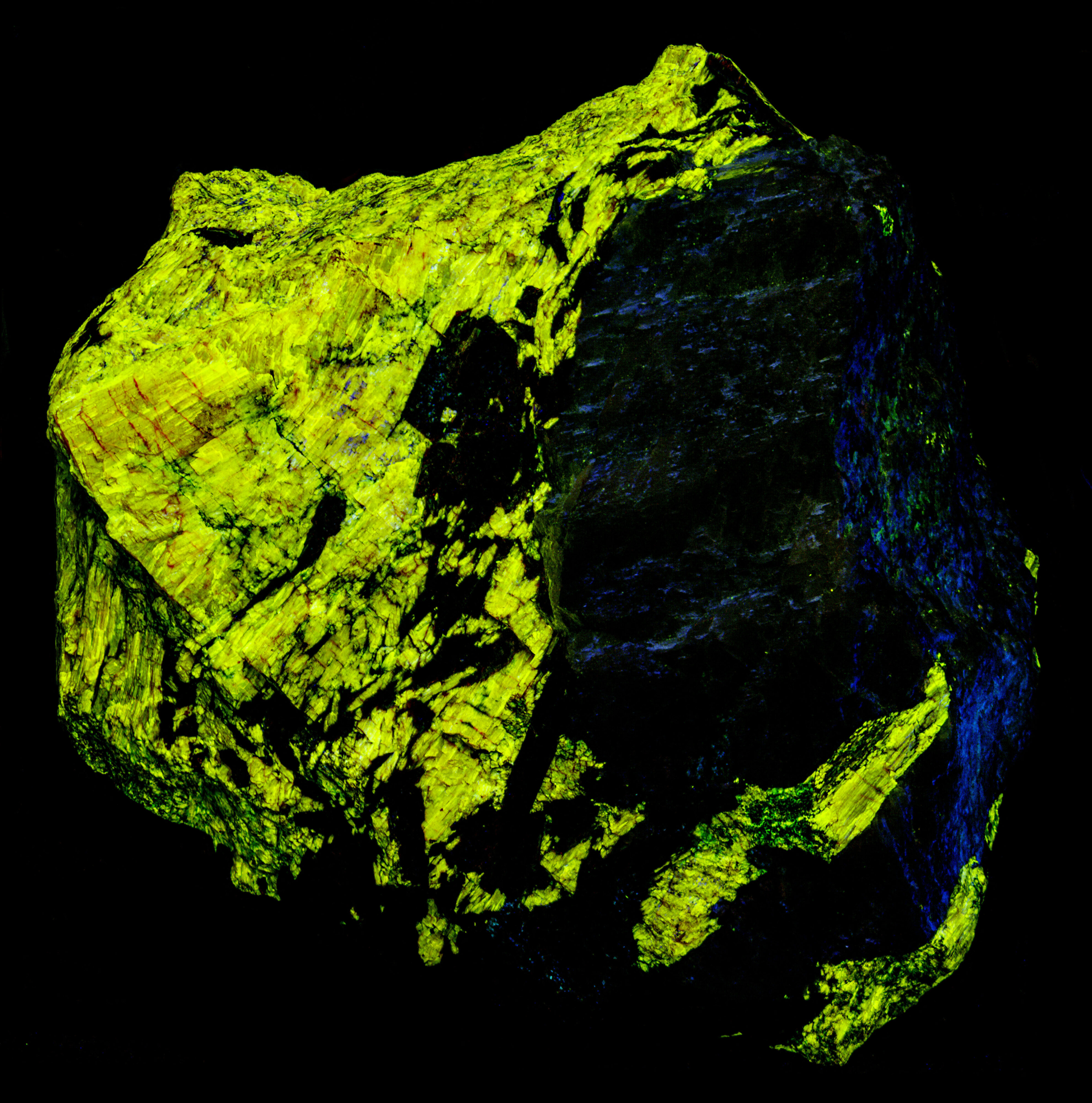
A false color image shows the bright ultraviolet fluorescence of the agrellite. This image is composed of three narrow band images that are shown on the spectral plot. The 310 nm band is assigned to blue, the 350 nm band is assigned to green, and the 394 nm band is assigned to red. The agrellite appears yellow in the false color image of ultraviolet fluorescence.
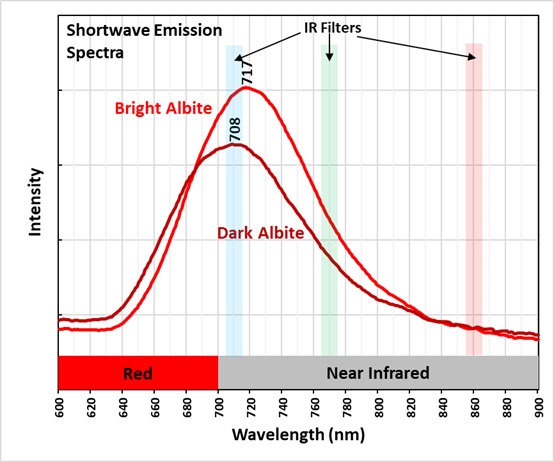
Albite fluoresces red under shortwave UV light. This red fluorescence is activated by ferric iron (Fe3+) replacing aluminum. However, the false color image of the near infrared fluorescence shows narrow, parallel zones of brighter fluorescence in the albite crystal. Emission spectra of the bright areas of the crystal have a peak at 717 nm. Emission spectra of the darker areas have peaks shifted towards shorter wavelengths by several nanometers (714 nm to 708 nm). The difference in the feldspar fluorescence may be caused by different concentrations of the ferric iron activator or a change in potassium versus sodium content in the albite.
Summary of luminescence responses:
Agrellite (Mindat) (RRUFF)
- Fluorescence under Shortwave (255nm LED) UV light: Pink
- Fluorescence under Midwave (305nm LED) UV light: Pink
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Fluorescence under Shortwave (255nm LED) UV light: Red