Celestine Crystal from Lime City, Wood County, Ohio
Contributed by: Michael Crawford
Date: Sep 18th, 2025
Locality: Stoneco Lime City Quarry, Lime City, Wood County, Ohio, USA (See on Mindat)
Size: 8 x 11 cm
Description:
A large celestine (SrSO4) crystal in a dolomite matrix from Lime City, Wood County, Ohio. This specimen was extracted from a quarry in the Greenfield Dolomite. The celestine crystal fluoresces bluish white under all wavelengths of UV illumination.
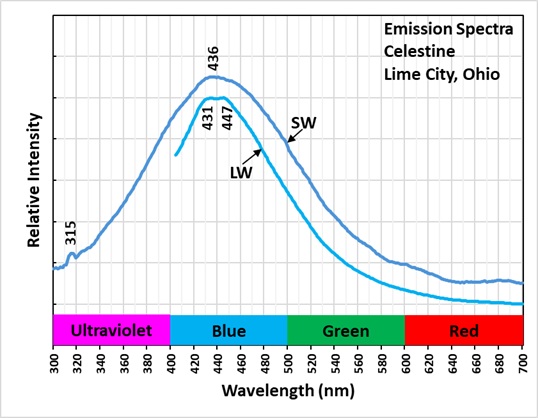
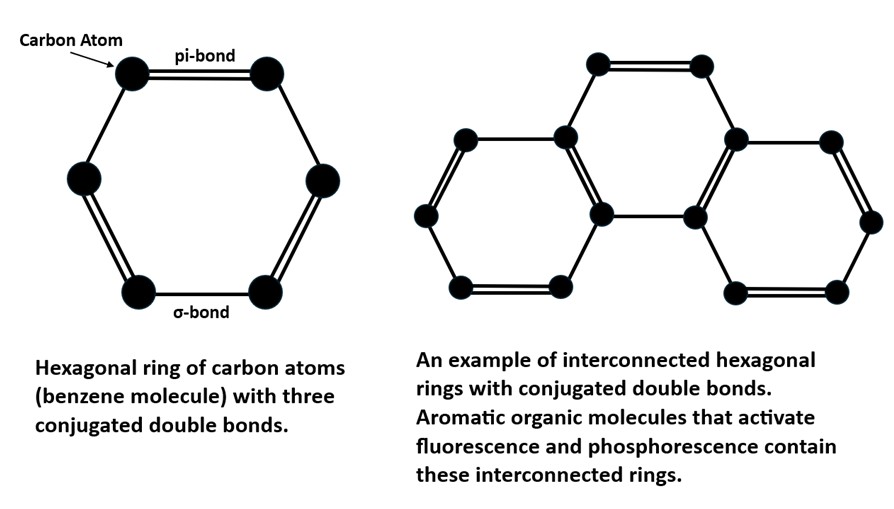
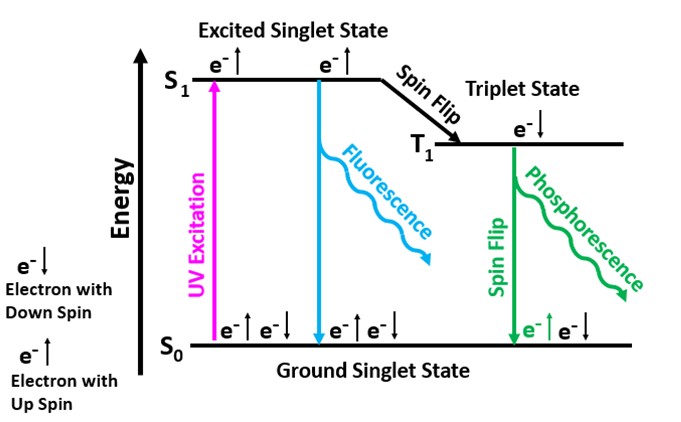
Both shortwave and longwave emission spectra peak around 440 nm. The longwave spectrum has two peaks at 431 nm and 447 nm. The crystal has very long-lasting afterglow from exposure to UV light. The longwave emission spectrum and the green afterglow indicate activation by aromatic organic molecules. These aromatic molecules contain interconnected hexagonal rings of carbon atoms with alternating single and double bonds. Electrons in the double bonds or pi-bonds of the rings are easily moved from the ground singlet state to an excited singlet state at a higher energy level by exposure to UV light. According to quantum mechanics, an electron orbital can contain either one or two electrons in the ground singlet state. If there are two electrons in the orbital, they must have opposite spins. In aromatic molecules, the singlet state contains two electrons with opposite spins and when excited by UV light one electron moves to a higher energy level of an excited singlet state. Excited singlet states are short-lived, so most electrons return to the original ground singlet state and the excess energy is emitted as a photon. The photon emission is the bluish white fluorescence we see with a peak around 440 nm.
Some electrons move from the excited singlet state to a slightly lower energy level known as the triplet state. The electron spin is flipped in the move to the triplet state, and it now has the same spin as the electron left in the ground singlet state. Quantum mechanics forbids electrons of the same spin in the ground singlet state, so electrons in the triplet state must flip its spin to return to the ground singlet state. This is a very low probability event, so it takes much more time for an electron to return to the ground state. An electron must wait until the ambient thermal crystal lattice vibrations cause it to flip again before it can fall back down to its original state and emit a photon. The triplet state is at a lower energy level compared to the excited singlet state. Therefore, the energy of the photon emitted when the electron moves back to the ground singlet state from the triplet state is less. According to Planck’s Law, lower photon energy corresponds to a longer wavelength (green) for the phosphorescence. Temperature affects the time for the spin to flip and for the electron to return from the triplet state to its original singlet state. Cooling the crystal extends the phosphorescent time.
This type of fluorescence and phosphorescence activated by aromatic organic compounds occurs in several minerals that form in near surface, low temperature environments. The list of minerals includes calcite, aragonite, selenite, celestine, fluorite, witherite, and colemanite.
Summary of luminescence responses:
Celestine (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Blue
- Fluorescence under Shortwave (255nm LED) UV light: Blue