Four-Color Specimen from the Garpenburg Mine, Sweden
Contributed by: Michael Crawford
Date: Sep 7th, 2025
Locality: Garpenberg Norra Mine, Garpenberg, Hedemora, Dalarna County, Sweden (See on Mindat)
Size: 8.5 x 12 cm
Description:
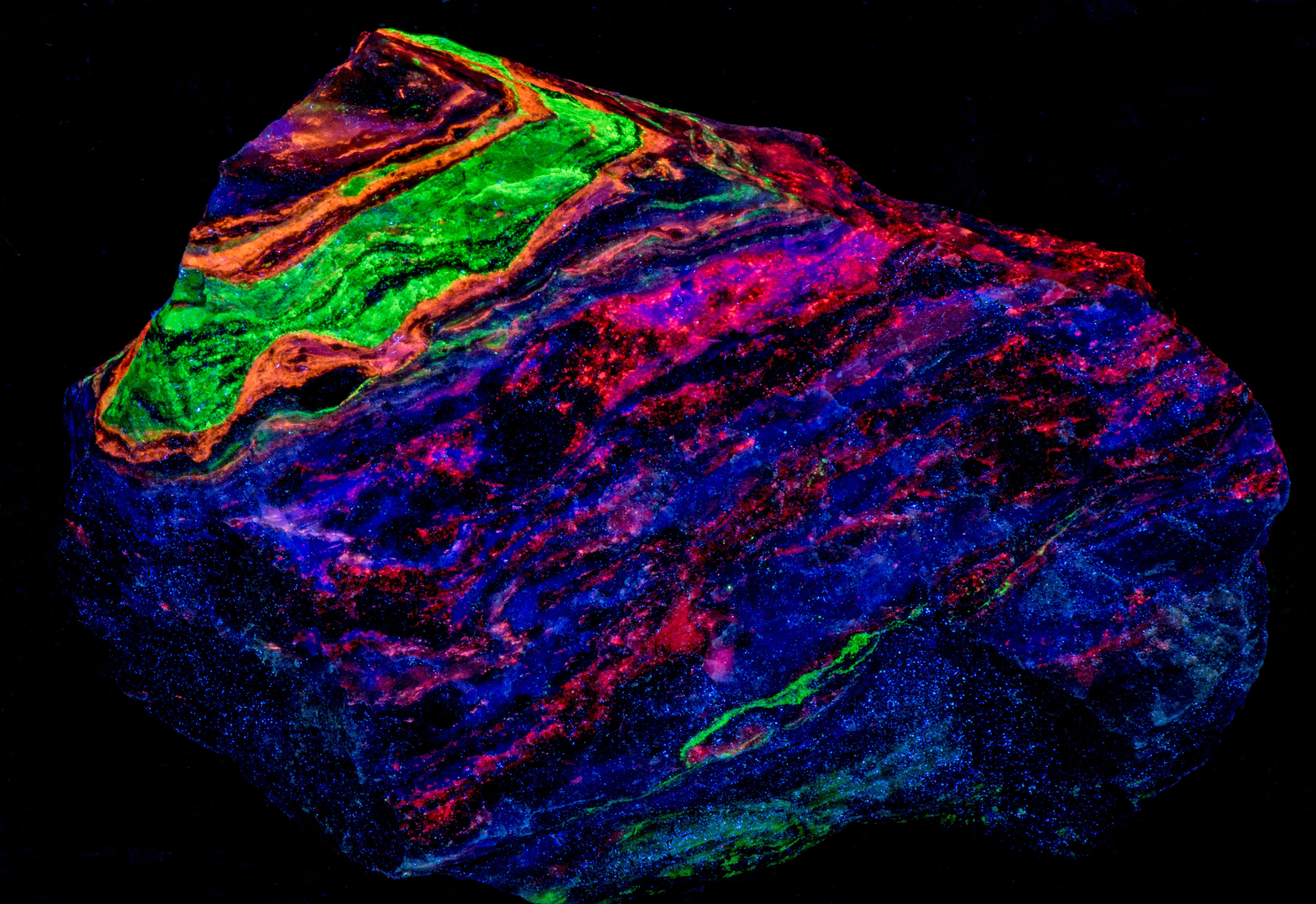
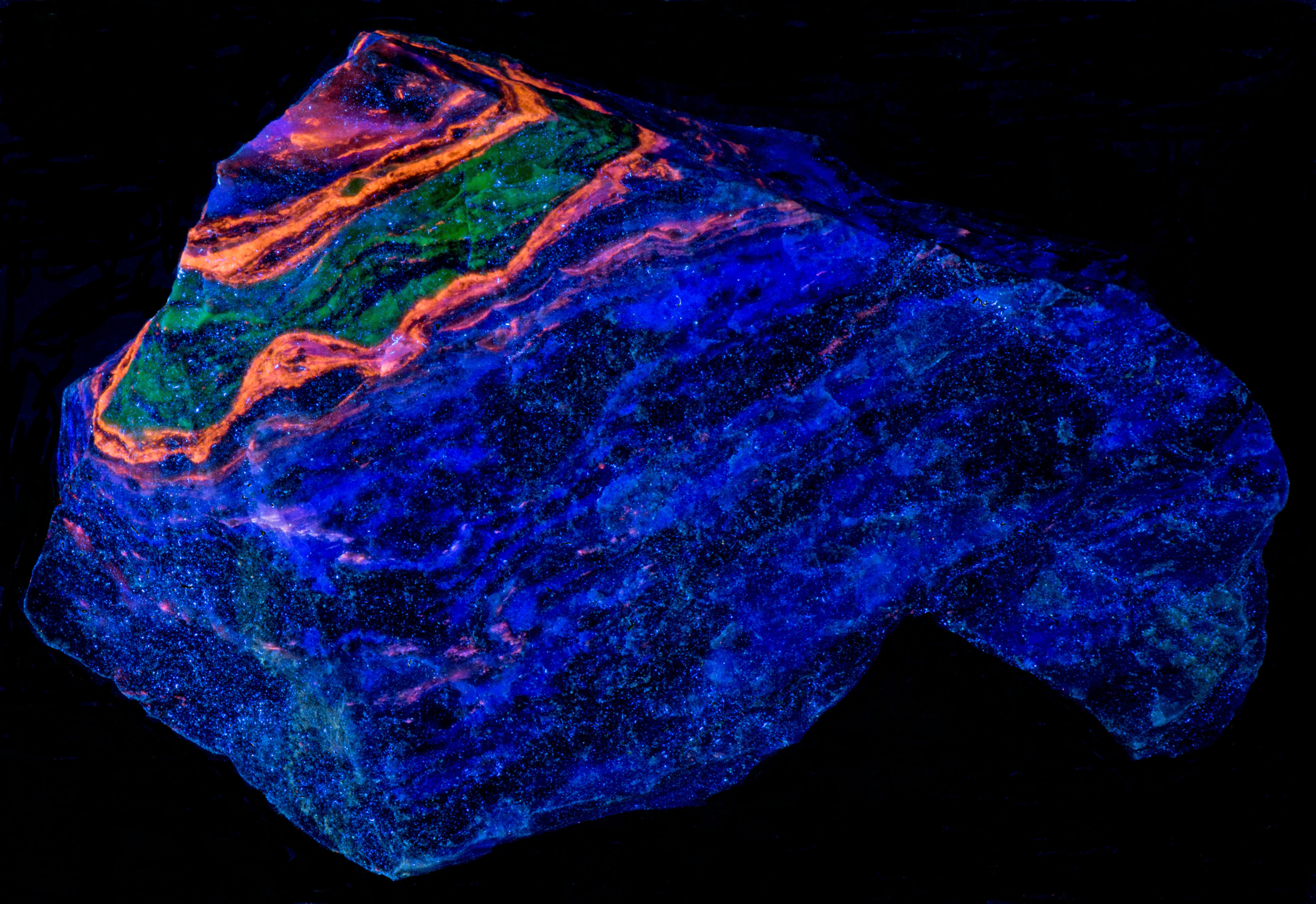
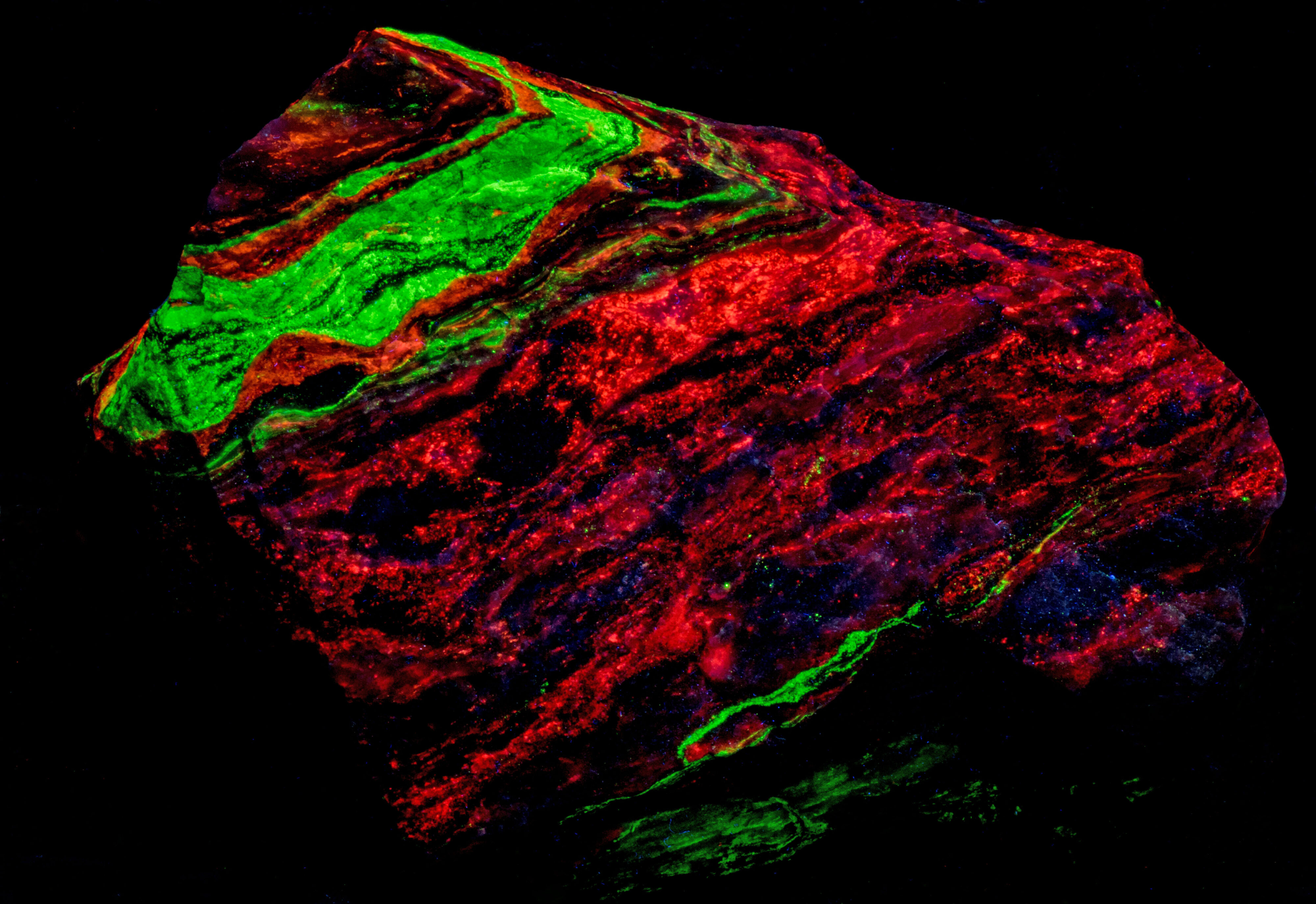
A four-color specimen from the Garpenberg Norra Mine, Garpenberg, Hedemora, Dalarna County, Sweden. The specimen contains the fluorescent minerals sphalerite, willemite, fluorite, and calcite. The four minerals occur in bands with one of the fluorescent minerals dominating a band. The emission spectra indicate that fluorite is mixed in with the other fluorescent minerals. Sphalerite fluoresces orange under all wavelengths of UV light. Willemite fluoresces bright green under shortwave UV light and is weakly fluoresces green under midwave and longwave UV light. Fluorite fluoresces blue only under longwave UV light. Calcite fluoresces red only under shortwave UV light.
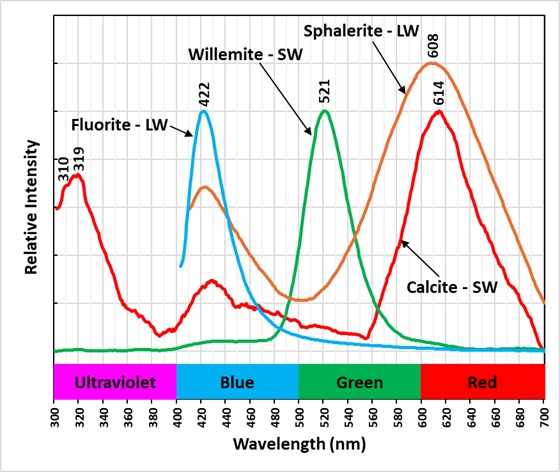
The last image shows the emission spectra of the minerals in the specimen. The longwave emission spectrum of the sphalerite has two peaks, one at 422 nm and 608 nm. The broad peak at 608 nm is caused by a manganese activator replacing some of the zinc. The 422 nm peak is caused by fluorite mixed in with the sphalerite. There is a small peak in the calcite shortwave spectrum at 422 nm due to fluorite mixed with the calcite. The calcite also has a peak in the red at 614 nm that is activated by manganese replacing calcium. The calcite has a third peak in the ultraviolet at 319 nm and an inflection point at 310 nm. This double peak is due to lead in the calcite (310 nm) and cerium in fluorite (319 nm).
Europium activates the blue longwave fluorescence in fluorite. Europium replaces calcium in the fluorite structure. The fluorite fluorescence peaks at 422 nm. Willemite fluoresces brightest under shortwave UV light. Manganese activates green fluorescence in willemite by replacing zinc. The willemite spectrum peaks at 521 nm.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Fluorescence under Shortwave (255nm LED) UV light: Orange
- Fluorescence under Longwave (365nm LED) UV light: Blue
- Fluorescence under Shortwave (255nm LED) UV light: Red
- Fluorescence under Shortwave (255nm LED) UV light: Green
- Fluorescence under Longwave (365nm LED) UV light: Green