Sphalerite, Fluorite and Willemite from the Garpenburg Mine, Sweden
Contributed by: Michael Crawford
Date: Sep 7th, 2025
Locality: Garpenberg Norra Mine, Garpenberg, Hedemora, Dalarna County, Sweden (See on Mindat)
Size: 6.5 x 10.5 cm
Description:
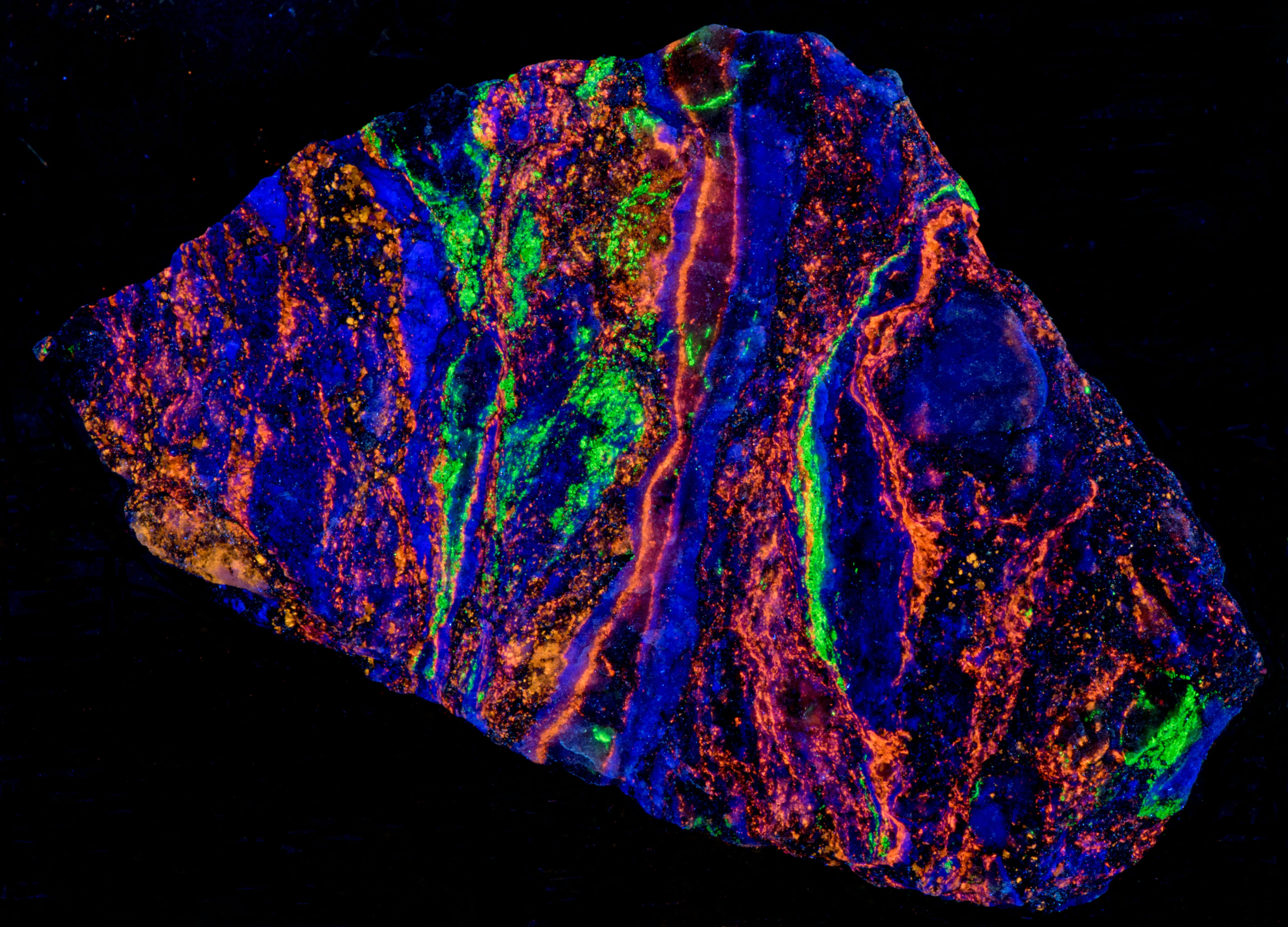
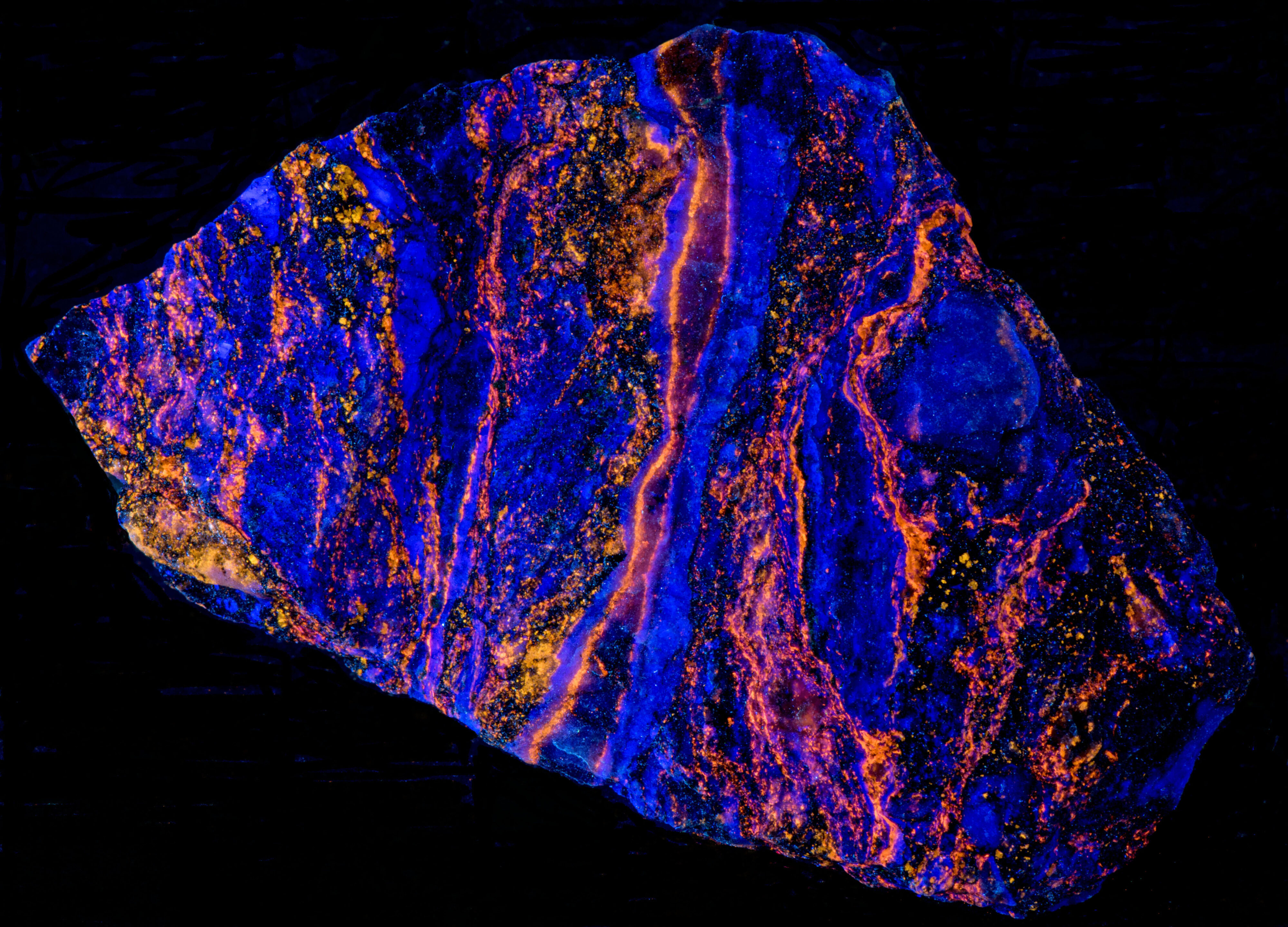
This specimen exhibits a variation of the fluorescent properties of sphalerite specimens from the Garpenberg Norra Mine, Garpenberg, Hedemora, Dalarna County, Sweden. The sphalerite in this specimen is phosphorescent exposure to longwave illumination. The phosphorescence has the same orange color as the fluorescence. The majority of the sphalerite specimens from Garpenberg that I own are non-phosphorescent. The sphalerite in this specimen fluoresces in all wavelengths, but the phosphorescence only occurs after exposure to longwave light. There is no phosphorescence from midwave and shortwave exposure. The phosphorescence may be caused by crystal lattice defects that trap the UV excited electrons and slowly releases them.
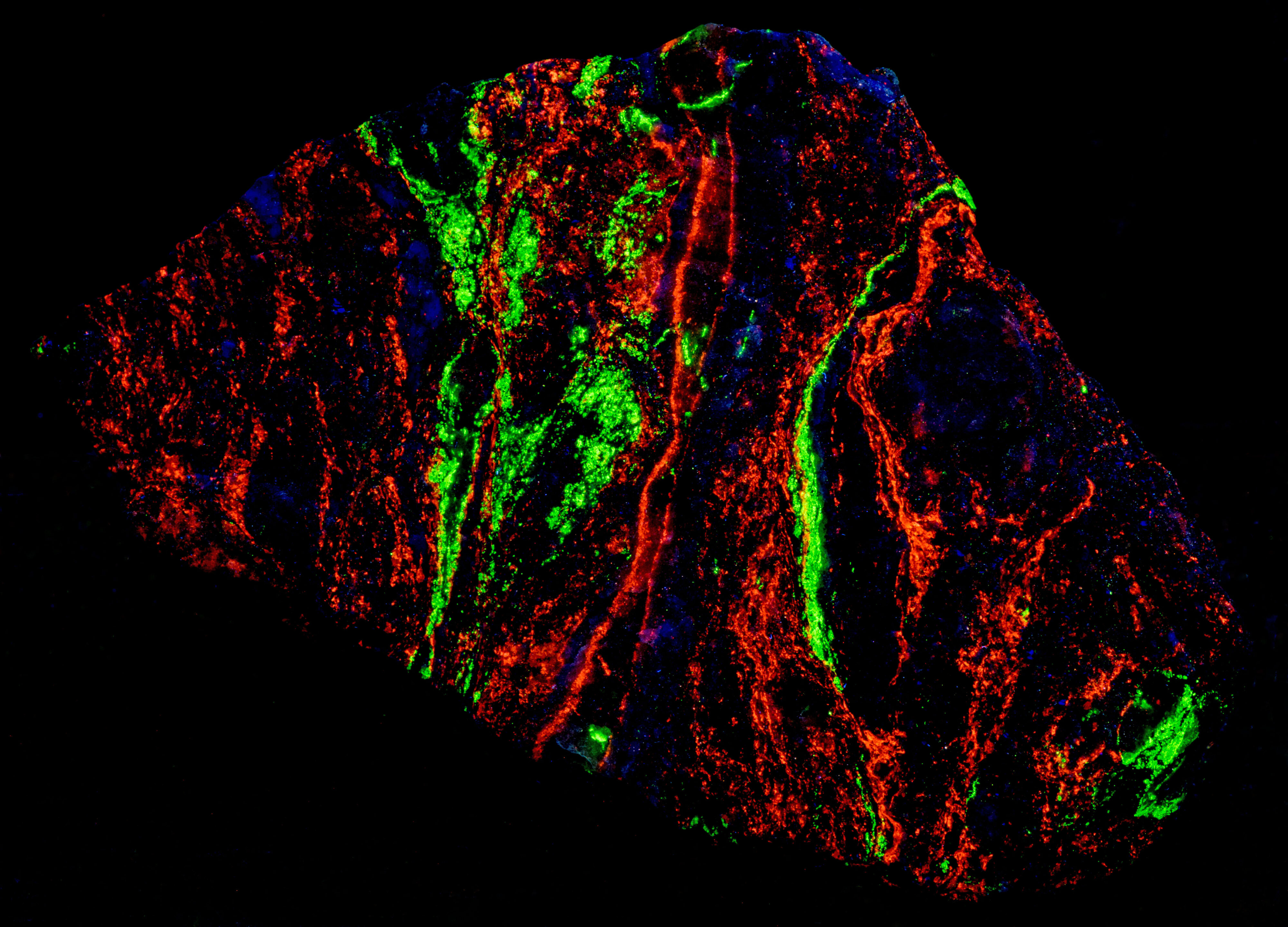
The other fluorescent minerals in this specimen are fluorite that fluoresces blue under longwave UV illumination and willemite that fluoresces green under shortwave illumination. The three fluorescent minerals generally occur in their own separate band in the specimen.
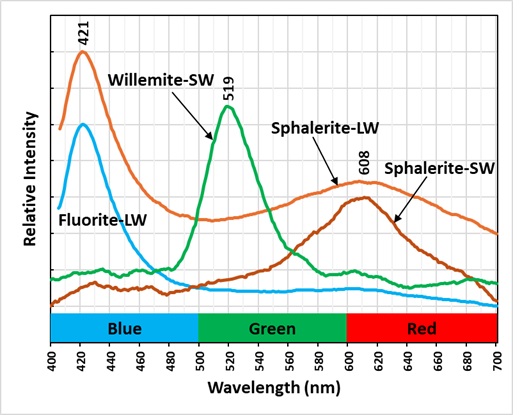
The last picture shows the emission spectra of the minerals in the specimen. The longwave emission spectrum of the sphalerite has two peaks, one at 421 nm and 608 nm. The broad peak at 608 nm is caused by a manganese activator replacing some of the zinc. The 421 nm is caused by fluorite mixed in with the sphalerite. The fluorite peak disappears in the shortwave spectrum of the sphalerite. The fluorescence of the fluorite also disappears under shortwave UV illumination. Europium activates the blue fluorescence in fluorite. Europium replaces calcium in the fluorite structure. Manganese also activates fluorescence in the willemite by replacing zinc.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Afterglow after exposure to Longwave (365nm LED) UV light: Orange
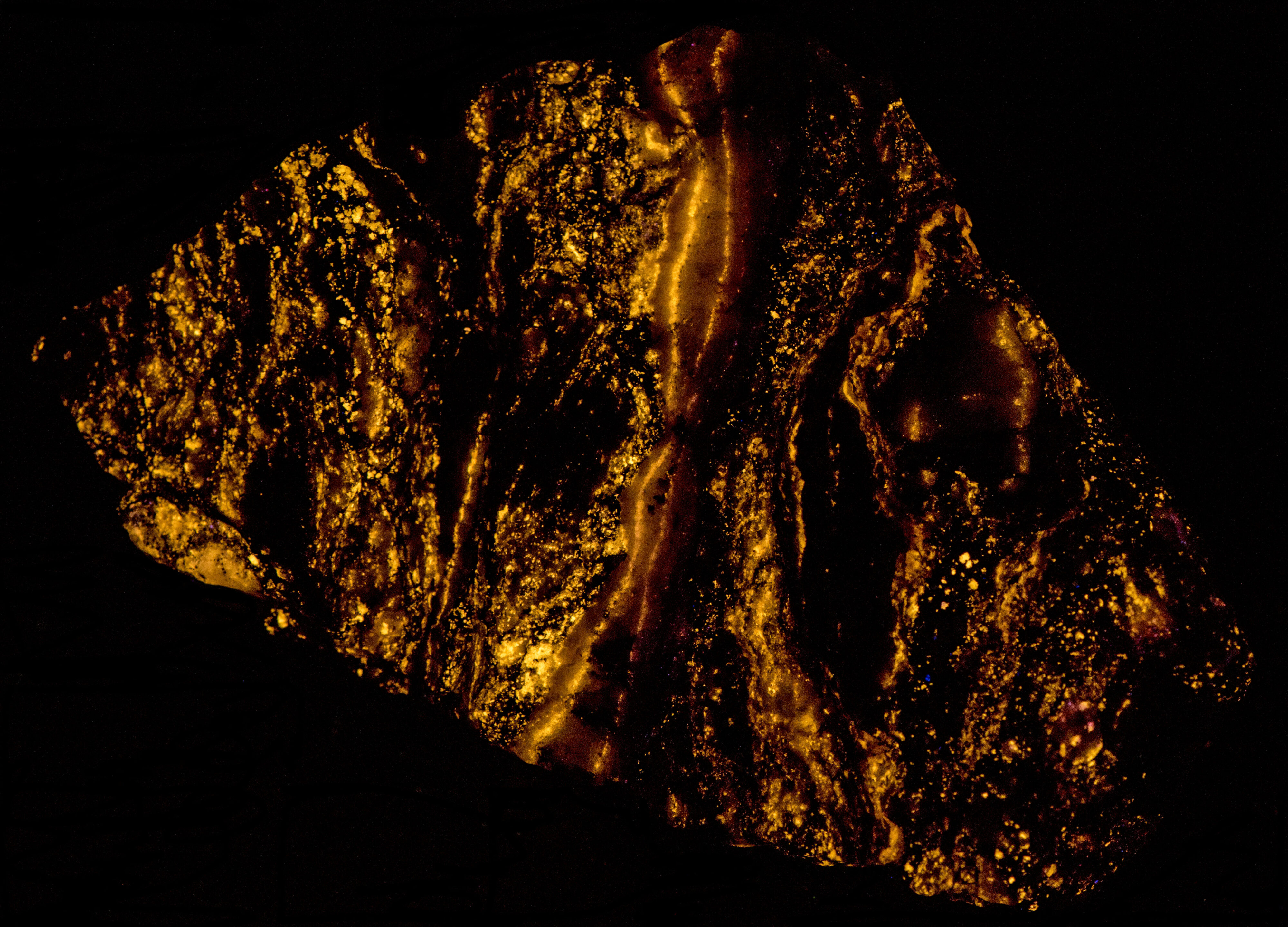
- Fluorescence under Shortwave (255nm LED) UV light: Orange
- Fluorescence under Longwave (365nm LED) UV light: Blue
- Fluorescence under Shortwave (255nm LED) UV light: Green