Radiation Alteration of Sodalite from Afghanistan
Contributed by: Michael Crawford
Date: Aug 1st, 2025
Locality: Sar-e-Sang, Kuran wa Munjan District, Badakhshan, Afghanistan (See on Mindat)
Size: 25 x 36 mm
Description:
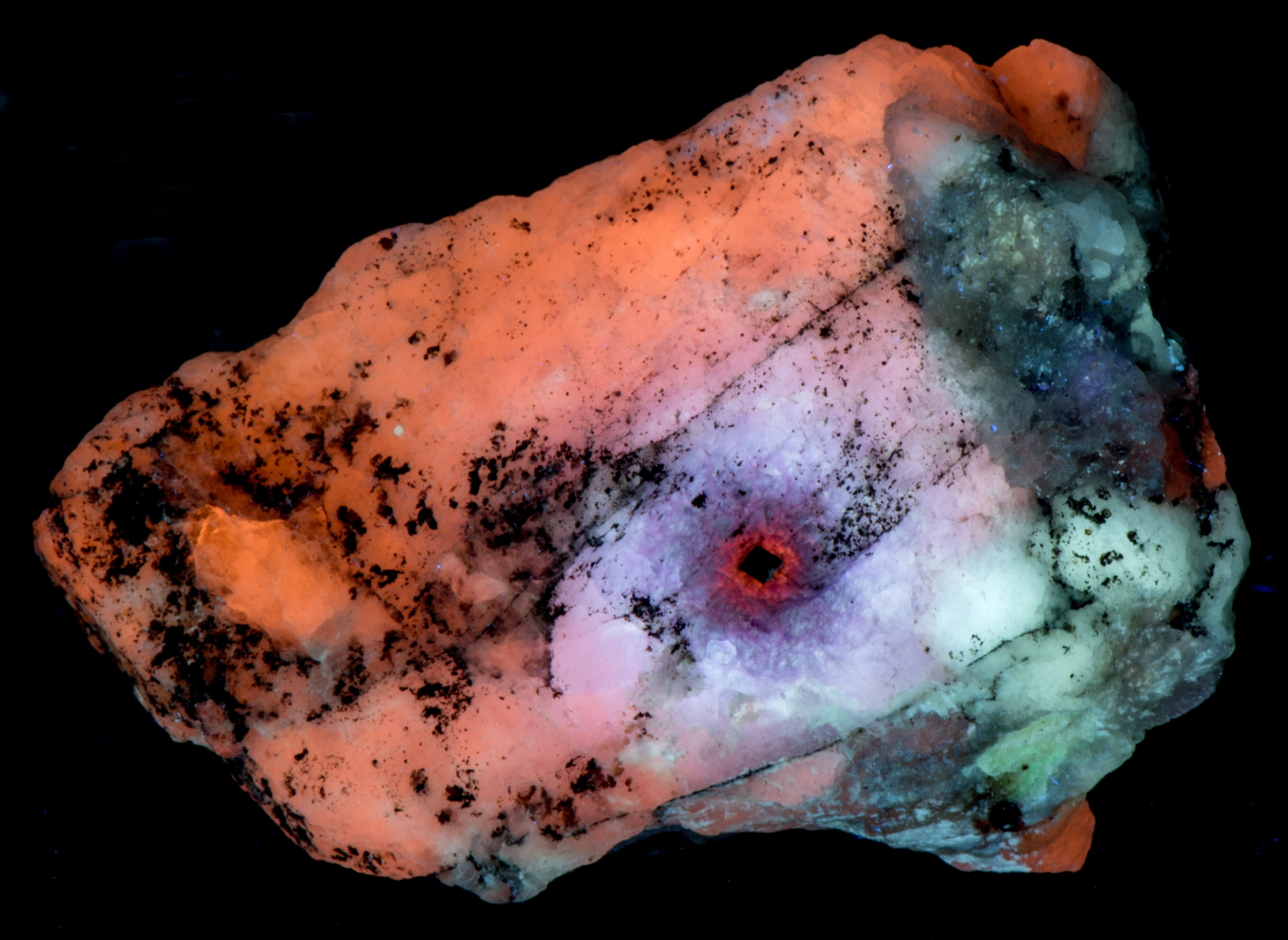
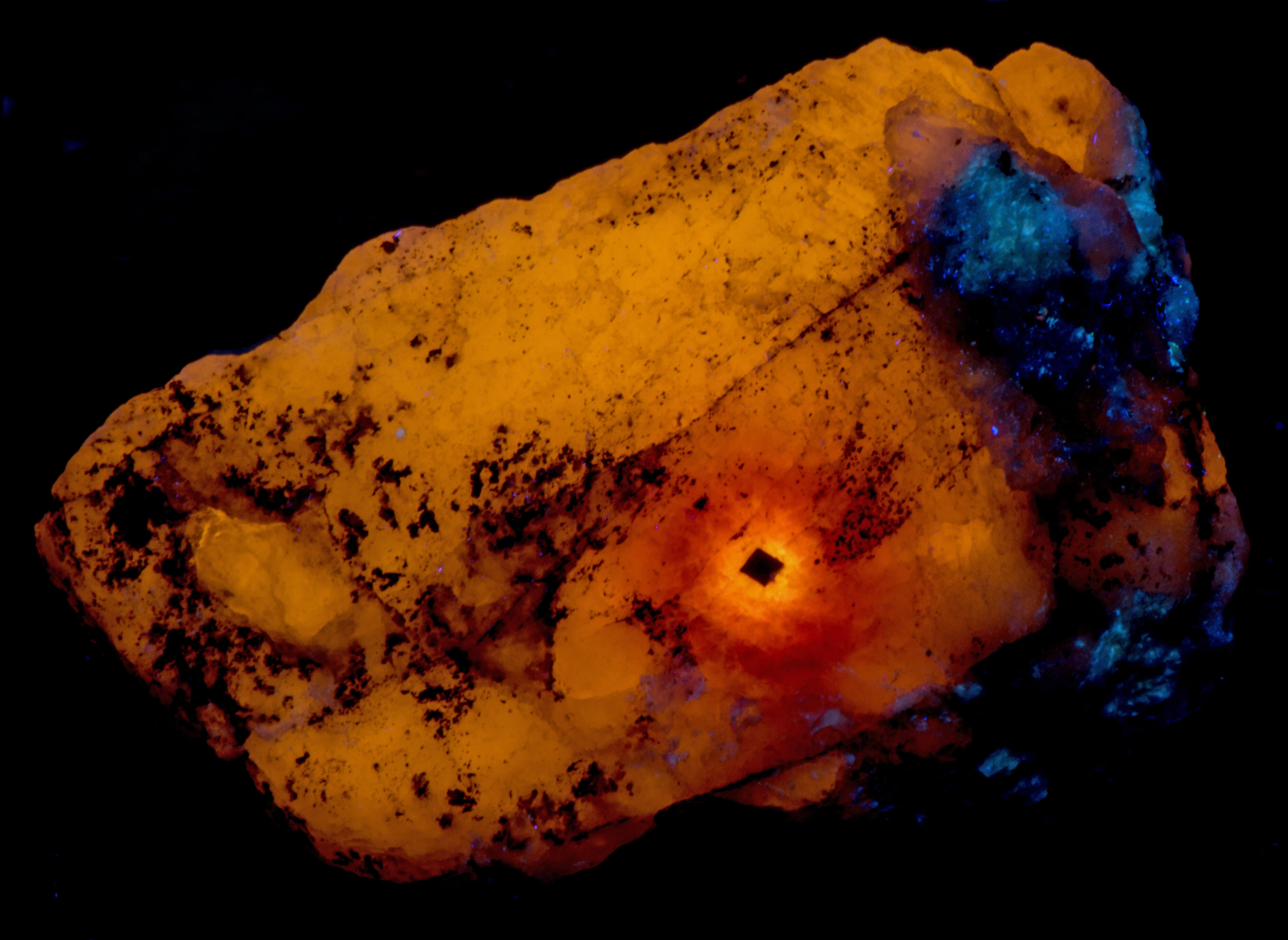
This is a thumbnail sodalite specimen from Sar-e-Sang, Badakhshan, Afghanistan that shows radiation alteration. This small specimen also shows four mechanisms that cause fluorescence, phosphorescence, and tenebrescence in sodalite. The radiation created one of these mechanisms (crystal defects) and modified the other mechanisms. Somehow radiation facilitated the migration and incorporation of titanium and iron activators in the original sodalite.
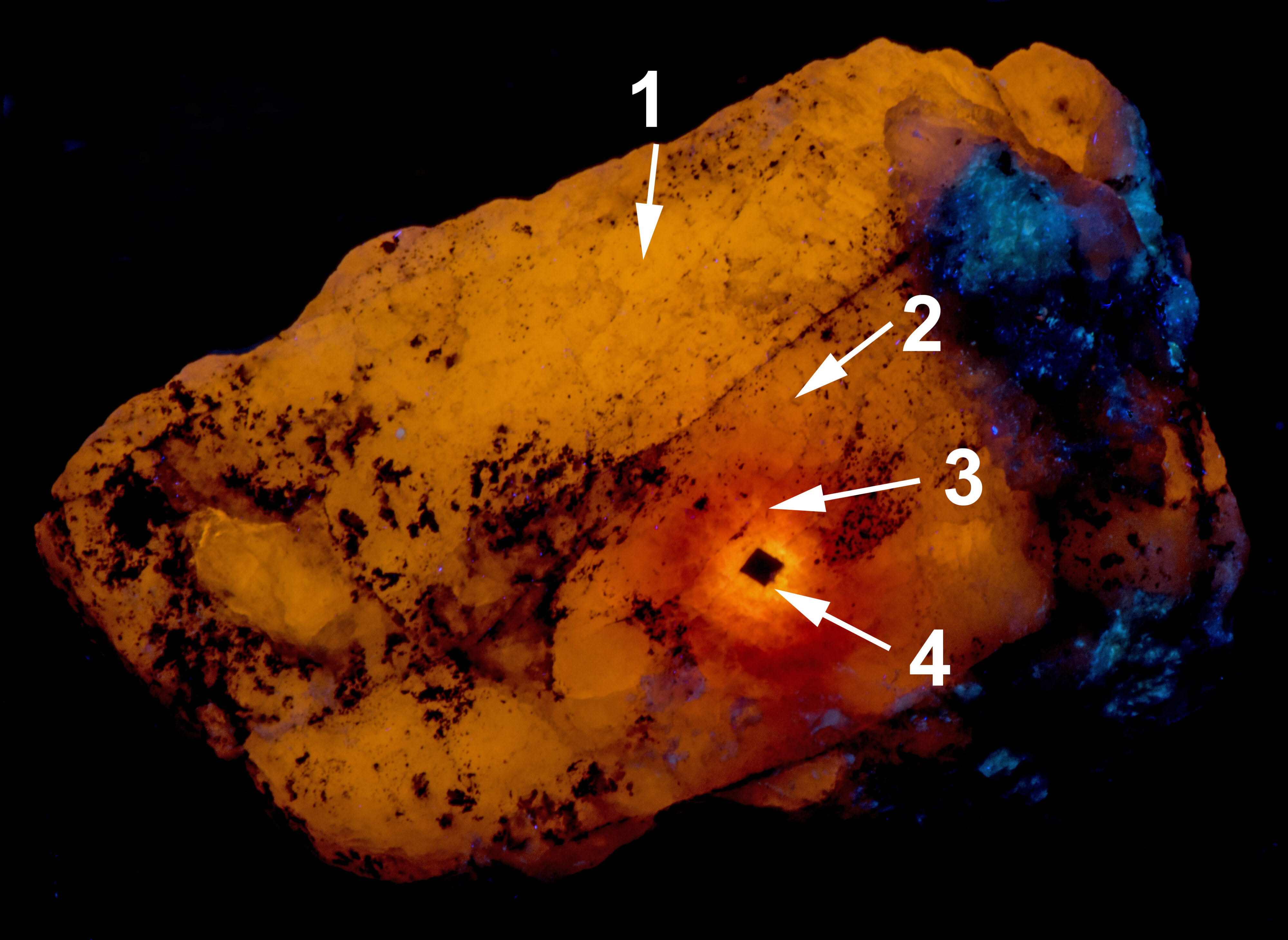
The longwave image shows a 1mm cubic crystal of uraninite surrounded by concentric zones of different fluorescent colors. The total count radiation at the crystal is around 200 cpm compared to a 20 cpm background. Other color changes caused by the radiation are also present in the midwave and shortwave fluorescence.
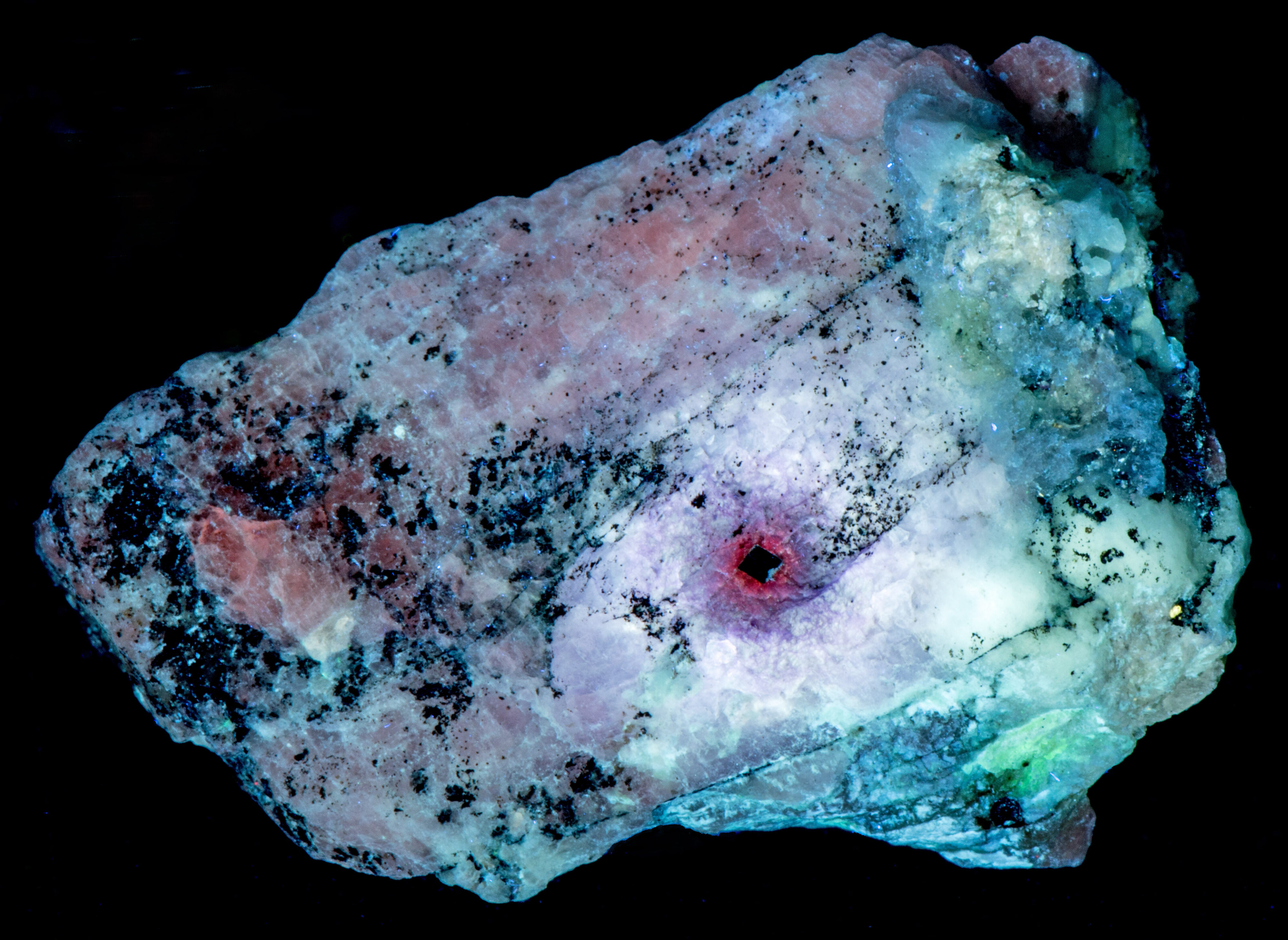
Other changes caused by radiation from the uraninite crystal include a loss of phosphorescence in the area adjacent to the uraninite crystal and stronger phosphorescence in the next zone away from the crystal. This same zone of stronger phosphorescence is also tenebrescent. Tenebrescence is caused by a crystal defect in sodalite. The radiation created the defect. The normal sodalite crystal lattice is a framework of linked AlO4 and SiO4 tetrahedra in which Si and Al alternate on the tetrahedral sites. The linkage forms a cage that contains a centrally located anion (chlorine (Cl-)) surrounded by four cations (sodium (Na+)) in tetrahedral coordination. The defect that causes tenebrescence occurs when chlorine anion is missing from the cage and an adjacent cage contains a disulfide anion instead of chlorine. The disulfide anion is the activator for the yellow-orange longwave fluorescence and it donates an electron that migrates into the adjacent empty cage when exposed to UV light. An electron in the cage creates a “F-center” or color center. When the sodalite is illuminated by white light, the color center absorbs green and some blue light and reflects red and the remaining blue light to produce the purple tenebrescent color. The tenebrescence fades as the electron migrates back to the disulfide anion and leaves the cage empty again destroying the F-center.
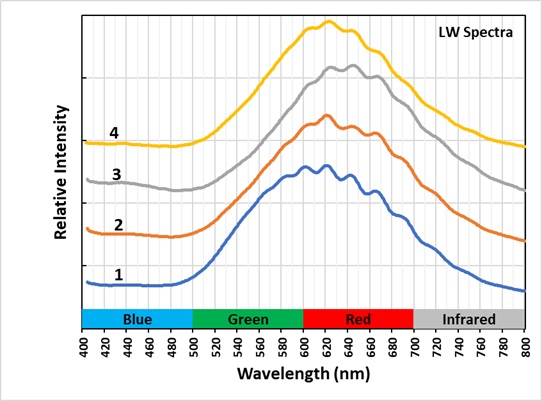
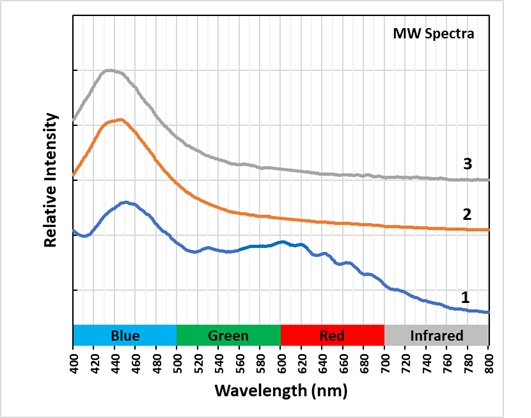
The last pictures show emission spectra of the different zones of alteration. The small peaks superimposed along the broad peak of the longwave spectra are vibronic peaks caused by vibration of the disulfide activator. The maximum of the longwave spectra changes for each zone. Shifts toward longer wavelengths are likely caused by increasing amounts of ferric iron (Fe3+) ions replacing aluminum in the sodalite structure and activating some red fluorescence. The iron is likely from trace amounts in the uraninite. The area next to the uraninite crystal fluoresces red in midwave and shortwave illumination. This red fluorescence is activated by Fe3+. I was not able to measure a spectrum of this red fluorescence.
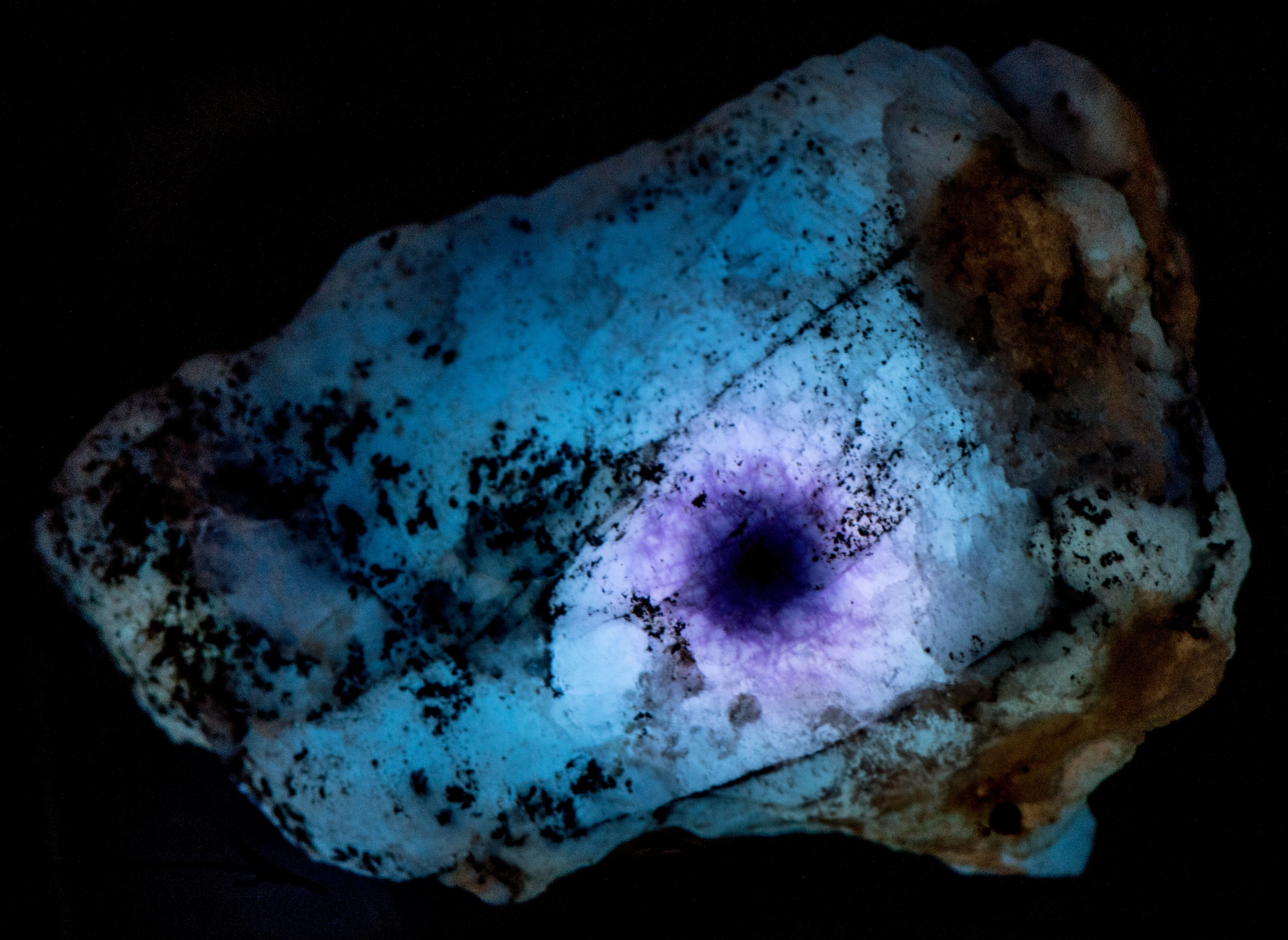
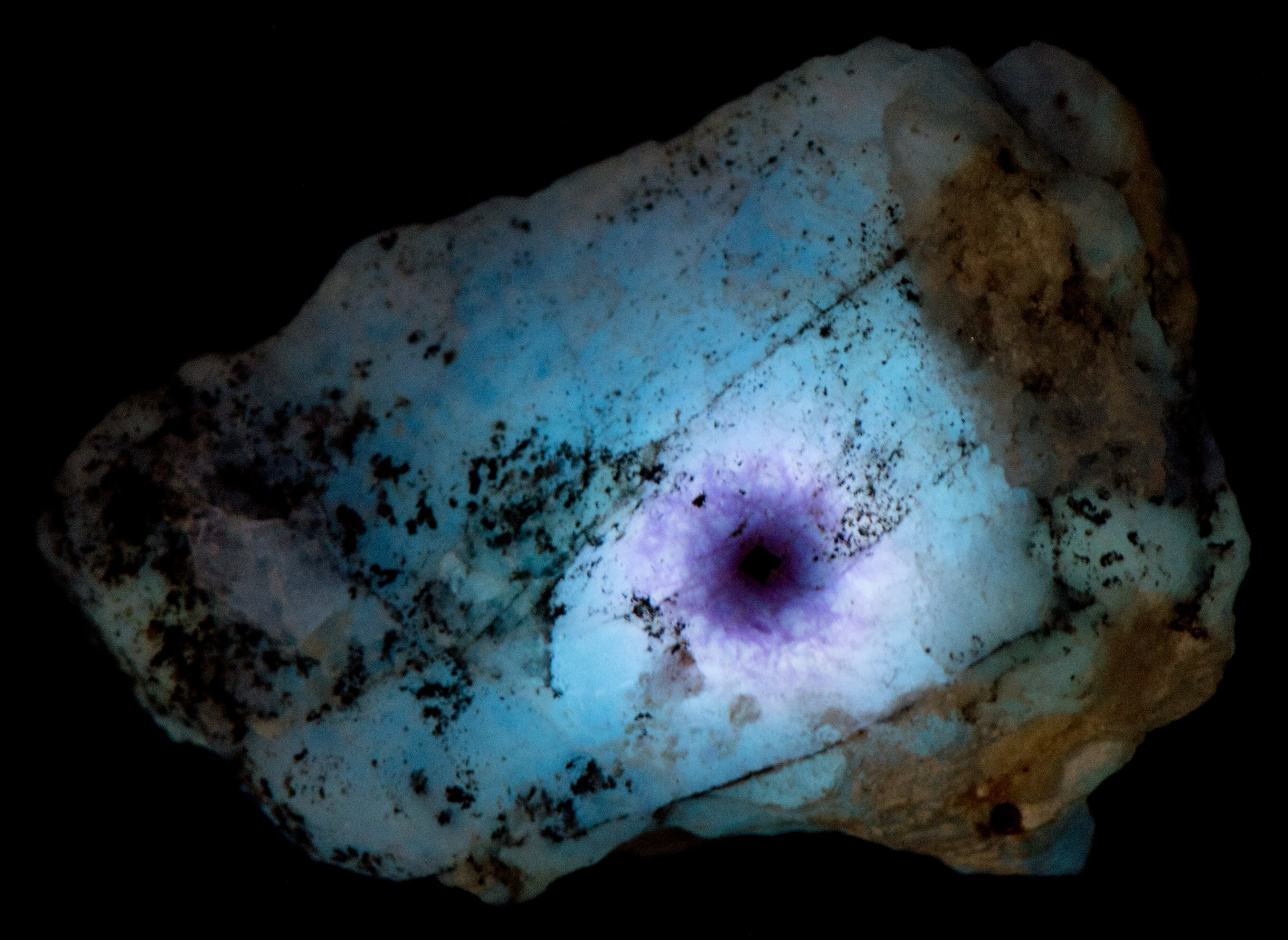
The midwave fluorescence of altered zones 2 and 3 is pinkish-white, and the shortwave fluorescence is bluish white. The emission spectra of midwave and shortwave fluorescence is dominated by a peak with a maximum around 440 nm to 450 nm. This fluorescence may be activated by titanium substituting for aluminum and a missing adjacent oxygen atom. The titanium substitution and oxygen vacancy are known to cause the strong blue-white afterglow seen in zones 2 and 3. Electrons are trapped in the oxygen vacancy during midwave and shortwave illumination, and they slowly return to their ground state causing the afterglow when the UV light is turned off. There is no afterglow in the area immediately adjacent to the uraninite crystal. The loss of afterglow may be caused by migration of titanium away from the crystal or quenching by iron.
The cause of midwave and shortwave fluorescence may also be activated by the titanium substitution for aluminum and the oxygen vacancy. The pinkish hue of the midwave fluorescence is from diminished fluorescence of the disulfide activated yellow-orange fluorescence combined with the blue-white fluorescence.
Summary of luminescence responses:
Sodalite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Fluorescence under Midwave (305nm LED) UV light: White
- Fluorescence under Shortwave (255nm LED) UV light: White
- Afterglow after exposure to Midwave (305nm LED) UV light: White
- Afterglow after exposure to Shortwave (255nm LED) UV light: White
- Tenebrescence after exposure to Shortwave (255nm LED) UV light: Purple
- Tenebrescence after exposure to Midwave (305nm LED) UV light: Purple
- Fluorescence under Longwave (365nm LED) UV light: Blue