Plumbian Orthoclase from Broken Hill, Australia
Contributed by: Michael Crawford
Date: Jul 28th, 2025
Locality: Broken Hill, Broken Hill district, Yancowinna Co., New South Wales, Australia (See on Mindat)
Size: 8 x 13.5 cm
Description:
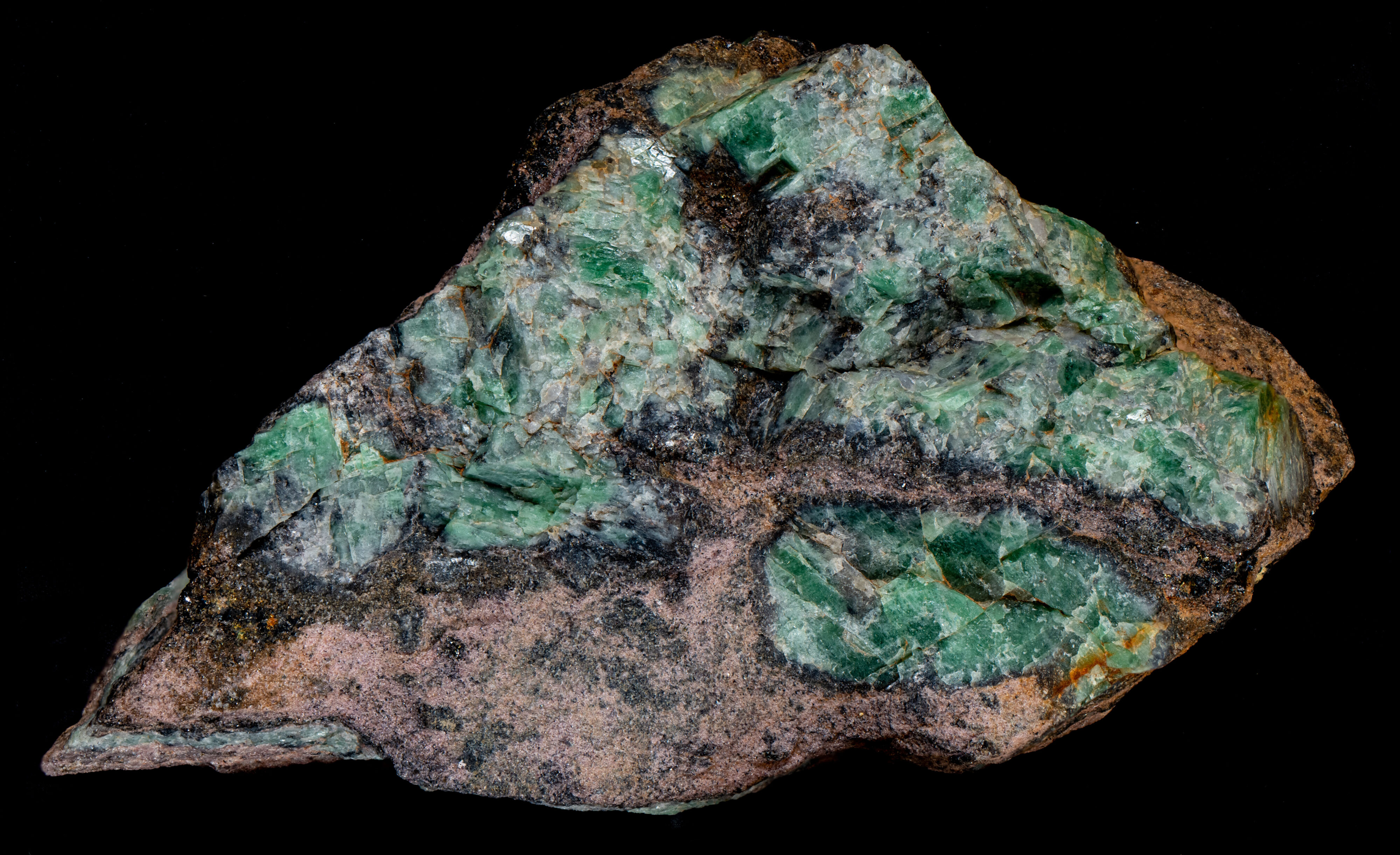
This is a specimen of green plumbian feldspar (K(AlSi3O8) ) from the North Mine, Broken Hill, Australia. This green feldspar is associated with the lead / zinc deposits and occurs at several areas in the Broken Hill district. Various references describe the green potassium feldspar from Broken Hill as both microcline and orthoclase, but most analysis favor the orthoclase identification. Microcline has a triclinic crystal structure and orthoclase has a monoclinic structure. Both have the same chemical formula and XRD or optical analysis is needed to distinguish them. The type of crystal structure depends on the temperature of formation, the rate of cooling and the corresponding rate of crystallization. Slow crystallization favors microcline development, orthoclase crystallization occurs more quickly, and sanidine, another K-feldspar, has the highest rate of crystallization.
The green color is attributed to a lead ion (Pb2+) replacing a potassium ion (K+) in the orthoclase crystal structure. An aluminum ion (Al3+) needs to replace a silicon ion (Si4+) to maintain charge balance. Exposure to sunlight affects the green color. Pale green pieces have turned deeper green on prolonged exposure.
This specimen contains a non-fluorescent, pink garnet. It has been called a “garnet sandstone” in some descriptions. Not sure if this is really a sedimentary formation, but it has the appearance of a sandstone. There are reaction rims of black sulfide minerals between the orthoclase and the garnet sandstone. I do not see silvery galena in the rims; the black color suggests sphalerite. There are also scattered grains of chalcopyrite in the reaction rims.
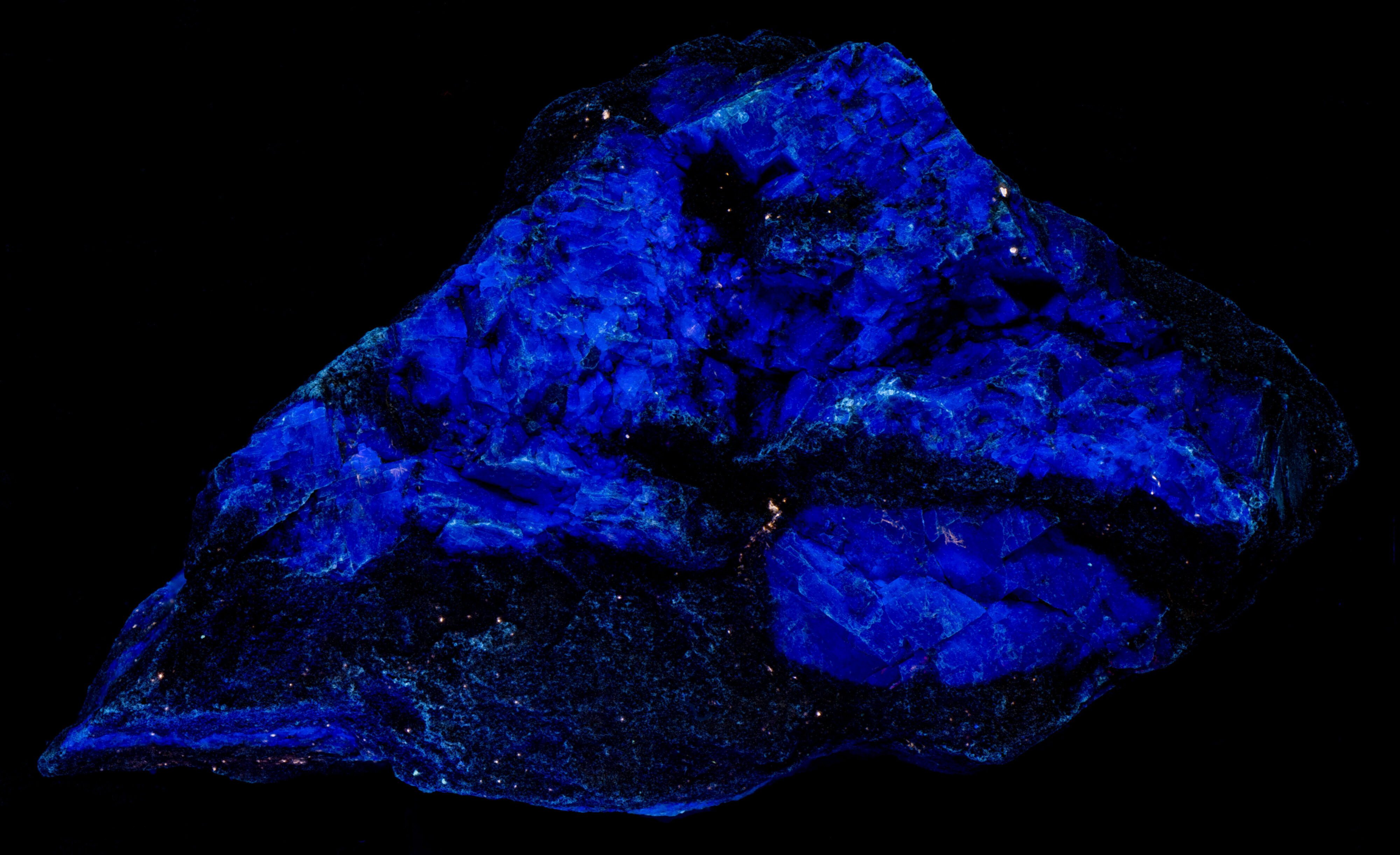
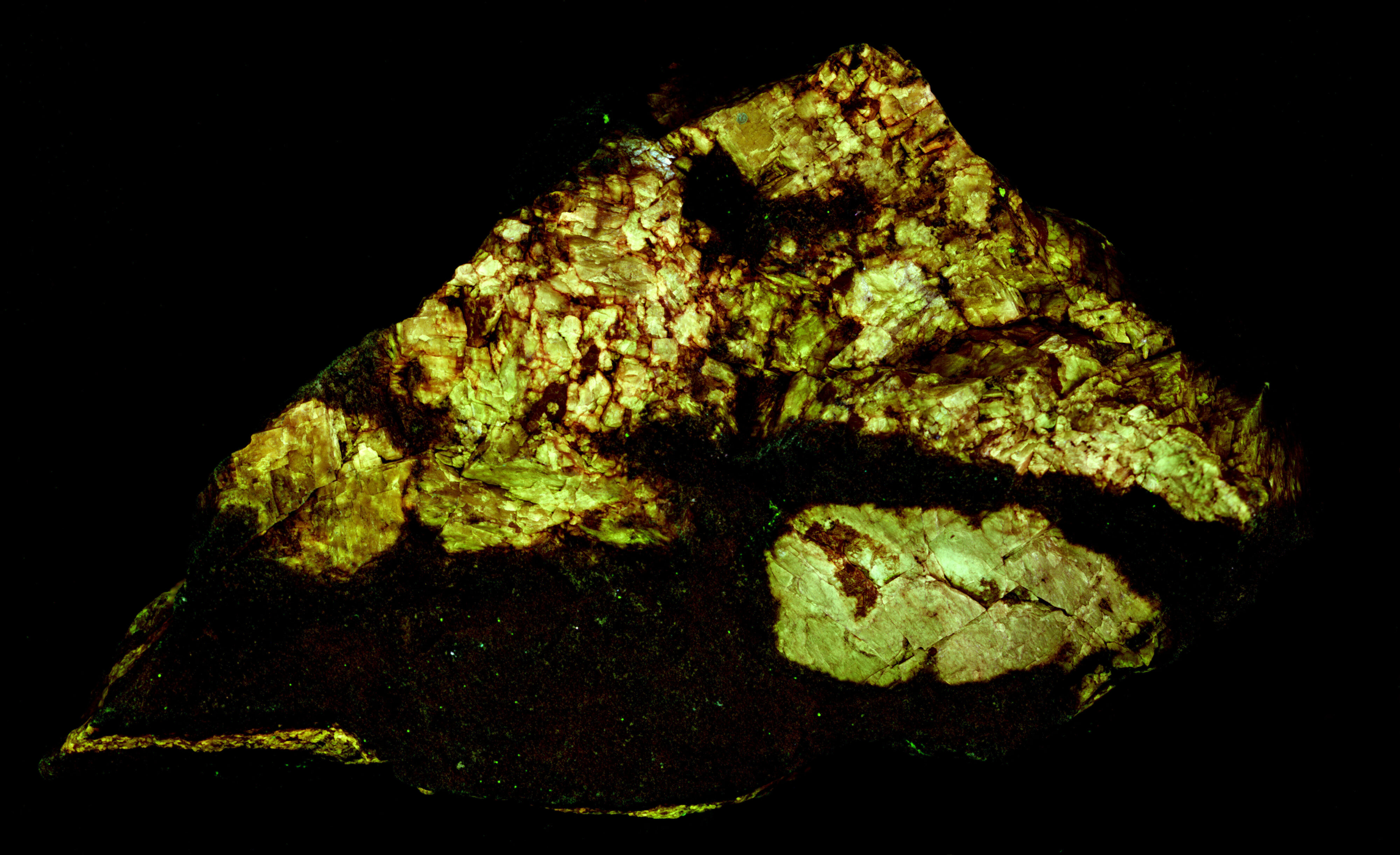
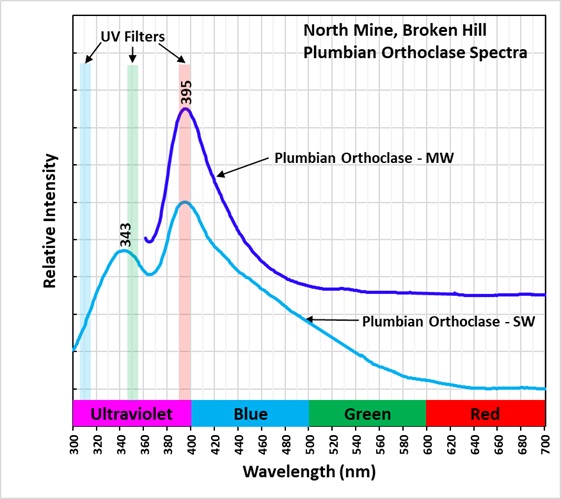
The shortwave emission spectra have two peaks in the ultraviolet region, one at 343 nm and another at 395 nm. I am not sure if lead is the activator for this ultraviolet fluorescence, or if there is another activator responsible for one of the peaks. The false color image shows the ultraviolet fluorescence of the orthoclase. There is also a broad peak in the blue and green visible region. Another activator causes this broad peak.
There is only one peak at 395 nm in the midwave emission spectrum and there is no broad peak in the blue and green visible region. The orthoclase fluorescence appears bluish white in the shortwave image because of the broad fluorescent peak in the visible blue and green. The orthoclase appears dark violet-blue in the midwave image because there is no blue-green visible fluorescence and only the longer wavelength shoulder of the 395 nm ultraviolet peak that extends into the visible violet and blue is imaged by the camera.
Summary of luminescence responses:
Orthoclase (Mindat) (RRUFF)
- Fluorescence under Midwave (305nm LED) UV light: Violet
- Fluorescence under Shortwave (255nm LED) UV light: White