Hauyne from Italy
Contributed by: Michael Crawford
Date: Jul 23rd, 2025
Locality: Ariccia, Metropolitan City of Rome Capital, Lazio, Italy (See on Mindat)
Size: 3.5 x 5 cm
Description:
A hauyne specimen from Ariccia, Metropolitan City of Rome Capital, Lazio, Italy. Hauyne is a member of the sodalite group. The sodalite mineral group has a framework of linked AlO4 and SiO4 tetrahedra in which Si and Al alternate on the tetrahedral sites. The linkage forms a cage that contains a centrally located anion surrounded by four cations in tetrahedral coordination. The sodalite group of minerals share the same alumino-silicate cage structure but have different anions and cations. Hauyne has a centrally located sulfate anion and three of the four cations in tetrahedral coordination are sodium and the fourth cation is calcium.
The endmember formulae for the sodalite group minerals are:
Sodalite -- Na4(Si3Al3)O12Cl
Hauyne -- Na3Ca(Si3Al3)O12(SO4)
Lazurite -- Na7Ca(Al6Si6O24)(SO4)(S3) · H2O
Nosean -- Na8(Al6Si6O24)(SO4) · H2O
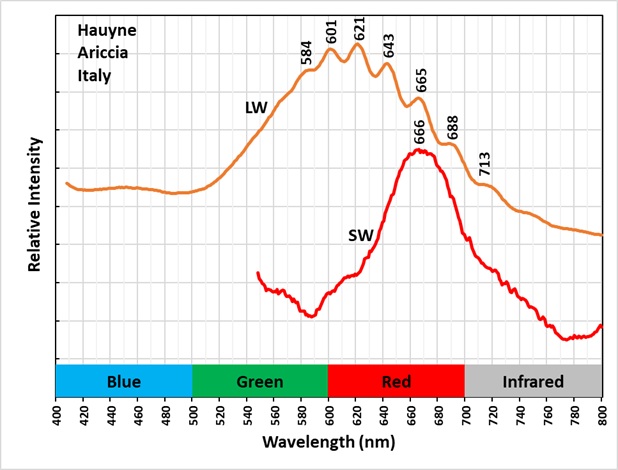
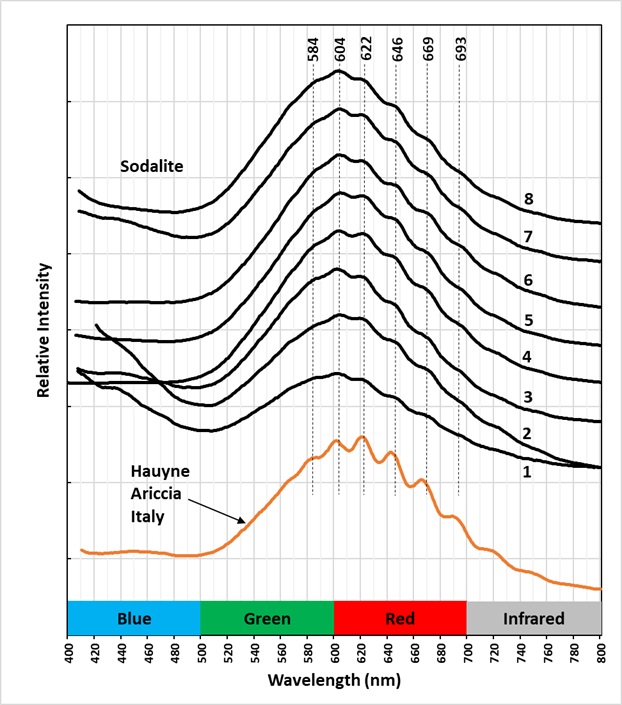
Hauyne fluoresces orange under longwave and midwave UV illumination. The fluorescence is activated by disulfide ions replacing the sulfate ion in the center of the alumino-silicate cage structure. When longwave UV light strikes hauyne; it causes the linear disulfide ion to vibrate. The resulting emission spectrum of this vibration is a broad peak with smaller peaks superimposed on the broad peak. These are called vibronic peaks. The amplitude of these vibronic peaks is much higher in hauyne compared to the vibronic peaks of sodalite. This higher amplitude is clearly shown in the spectral plots of sodalite specimens from several worldwide locations compared to this Italian hauyne specimen. The higher amplitude is likely caused by the alumino-silicate cage being larger due to the larger ionic sizes of calcium and sulfate in hauyne, compared to sodium and chlorine in sodalite.
The shortwave fluorescence under shortwave UV light is red. The shortwave spectrum is broad with a maximum at 666 nm. This red fluorescence is activated by ferric iron (Fe3+) replacing aluminum in the alumino-silicate cage structure.
Locations of Sodalite specimens used for emission spectral measurements shown in plot.
1 – Ice River Complex, British Columbia, Canada
2 – Sar-e-Sang, Badakhshan Province, Afghanistan
3 – Magnet Cove, Arkansas
4 – Illimausaq Complex, Greenland
5 – Mont St. Hilaire, Quebec, Canada
6 – Kola Peninsula, Murmansk Oblast, Russia
7 – Keweenaw Peninsula, Michigan
8 – Tvadalen, Norway
Summary of luminescence responses:
Hauyne (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Fluorescence under Midwave (305nm LED) UV light: Orange
- Fluorescence under Shortwave (255nm LED) UV light: Red