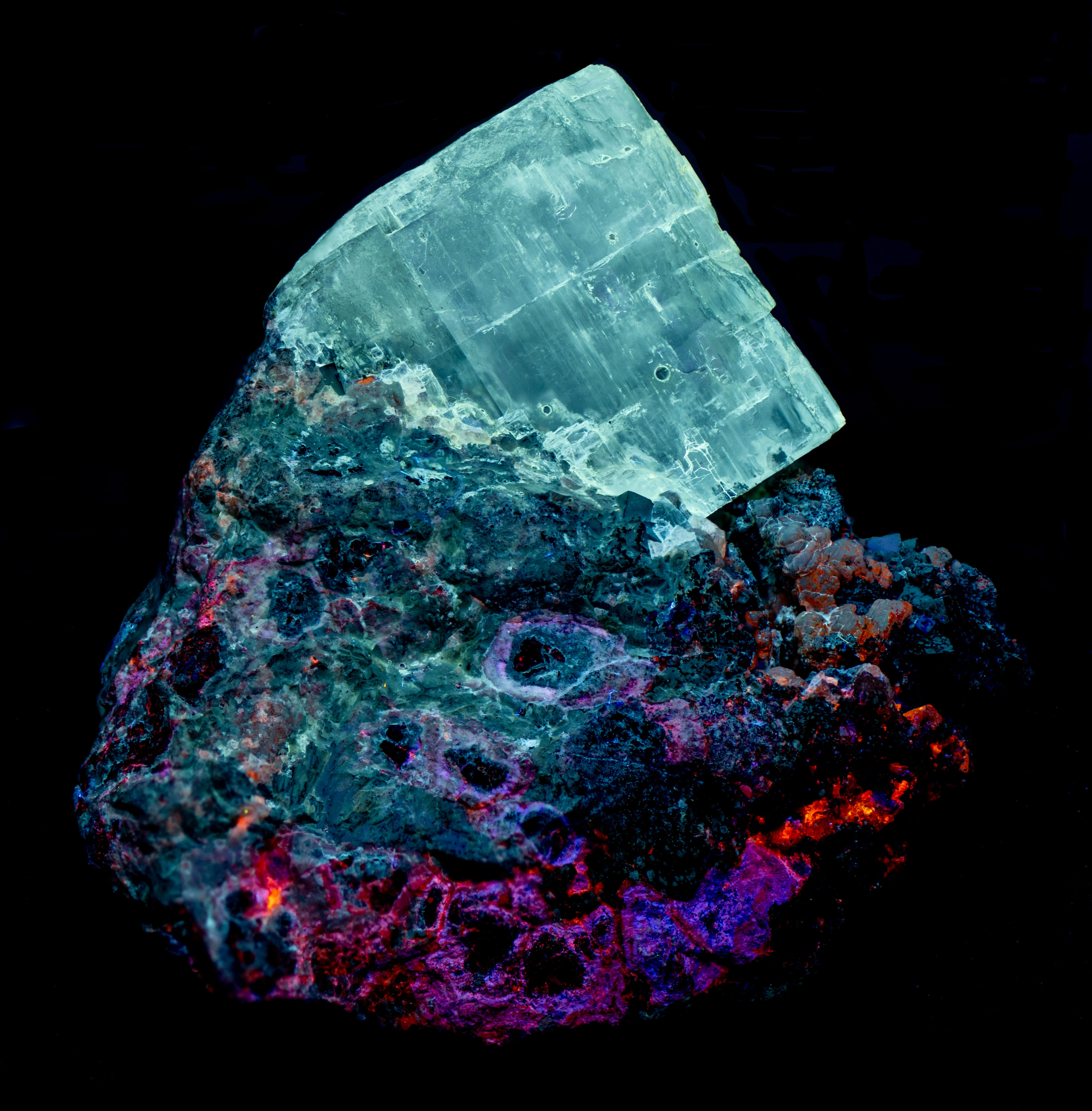
\"Bird\'s Eye\" Sphalerite and Anhydrite from Balmat, New York
Contributed by: Michael Crawford
Date: Jul 16th, 2025
Locality: Empire State No. 4 Mine, Balmat, Fowler, St. Lawrence County, New York, USA (See on Mindat)
Size: 30 x 40 mm
Description:
This is a thumbnail specimen of sphalerite, anhydrite, and magnetite from the 2,500 ft level of the ZCA #4 (Empire State) Mine, Balmat, St. Lawrence County, New York. The specimen exhibits the classic “bird’s eye” structure that I think is unique to this mine. The bird’s eye structure is a rounded grain of black magnetite surrounded by a halo of sphalerite. The sphalerite fluoresces violet-blue, pink and orange under longwave UV illumination and the magnetite is non-fluorescent. The color and fluorescent intensity of the sphalerite changes under midwave and shortwave illumination. The anhydrite crystal fluoresces white under all wavelengths.
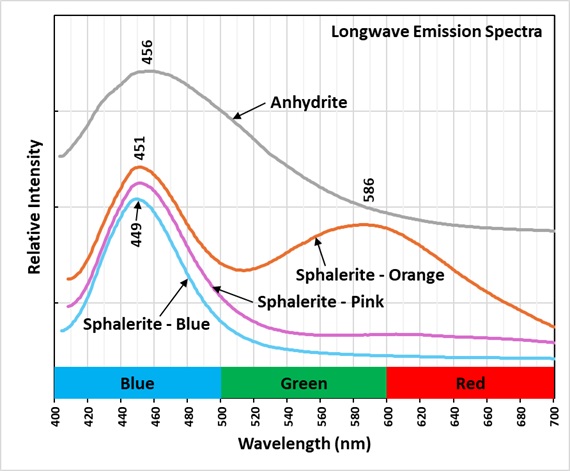
The longwave emission spectra of violet-blue, pink, and orange, fluorescent sphalerite all have peaks around 450 nm. The orange, fluorescent sphalerite has a second broad peak with a maximum at 586 nm. The violet-blue fluorescence is the result of UV light moving electrons from the valance band in sphalerite to its conduction band. When electrons return to the valance band violet-photons are emitted. Adding manganese to the sphalerite shrinks the amount of energy needed to move electrons out of the valance band. When these electrons return to the valance band, the photons emitted have less energy and thus are at a longer wavelength (orange). The pink fluorescence represents an intermediate level of manganese content that adds increasing amounts of orange fluorescence to the violet-blue fluorescence.
The anhydrite emission spectrum has a very broad peak with a maximum at 456 nm. The dioxide replacing sulfate is a possible activator for this fluorescence.
Summary of luminescence responses:
Sphalerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Violet
- Fluorescence under Longwave (365nm LED) UV light: Pink
- Fluorescence under Longwave (365nm LED) UV light: Orange
- Fluorescence under Longwave (365nm LED) UV light: White