Schrockingerite from Wyoming
Contributed by: Michael Crawford
Date: Jun 23rd, 2025
Locality: Lost Creek area, Sweetwater County, Wyoming, USA (See on Mindat)
Size: 5 x 6 cm
Description:
This is schrockingerite (NaCa3(UO2)(CO3)3(SO4)F · 10H2O) mixed with sandstone and gypsum from the Lost Creek area, Sweetwater County, Wyoming. The blue-green intrinsic fluorescence is caused by the uranyl ion being a part of the schrockingerite crystalline structure. The schrockingerite fluoresces blue-green under all wavelengths of UV illumination.
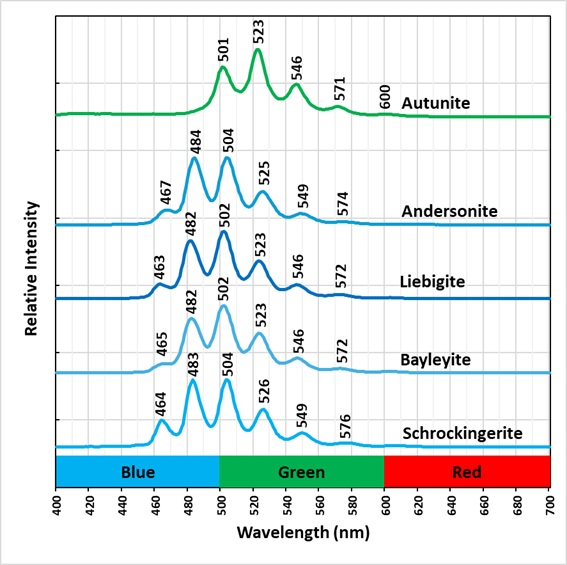
The uranyl ion has a linear structure with a hexavalent uranium atom between two oxygen atoms. Short uranium-oxygen distances between atoms indicate multiple bonds between uranium and oxygen. The uranium in the uranyl ion has an oxidation state of 6+, so there are two bonds with each oxygen atom leaving the uranyl ion with an oxidation state of 2+. There are 2 bonds between each oxygen atom and the uranium atom. Linear vibrations and bending of the atoms occur when exposed to ultraviolet illumination causing multiple peaks in the emission spectrum. The multiple peaks are called vibronic. The bonds between the uranyl ion and other elements in the mineral are perpendicular to the axis of the uranyl ion. The position and strength of the vibronic peaks depend on the coordination with the other atoms in the mineral.
The vibronic peaks of schrockingerite are shifted to shorter wavelengths compared to the vibronic peaks of autunite (see spectral plots). This shift is caused by the different coordination between the uranyl ion and carbonate ion in schrockingerite and the uranyl ion and phosphate ion autunite. The fluorescence of shrockingerite appears blue-green because of this shift compared to the yellow-green color of autunite fluorescence. Other uranyl, carbonate minerals also have blue-green fluorescence as shown in the spectral plots.
Schrockingerite (NaCa3(UO2)(CO3)3(SO4)F · 10H2O)
Bayleyite (Mg2(UO2)(CO3)3 · 18H2O)
Andersonite (Na2Ca(UO2)(CO3)3 · 6H2O)
Liebigite (Ca2(UO2)(CO3)3 · 11H2O)
The emission spectra indicate that the peak positions of uranyl-carbonate minerals vary slightly due to differences in coordination between different cations, anions, and the uranyl ion in each mineral. Emission spectra have the potential to assist in the identification of uranium minerals.
The specimen’s radioactivity is around 250 CPM.
Summary of luminescence responses:
Schrockingerite (Mindat) (RRUFF)
- Fluorescence under Longwave (365nm LED) UV light: Green
- Fluorescence under Midwave (305nm LED) UV light: Green
- Fluorescence under Shortwave (255nm LED) UV light: Green